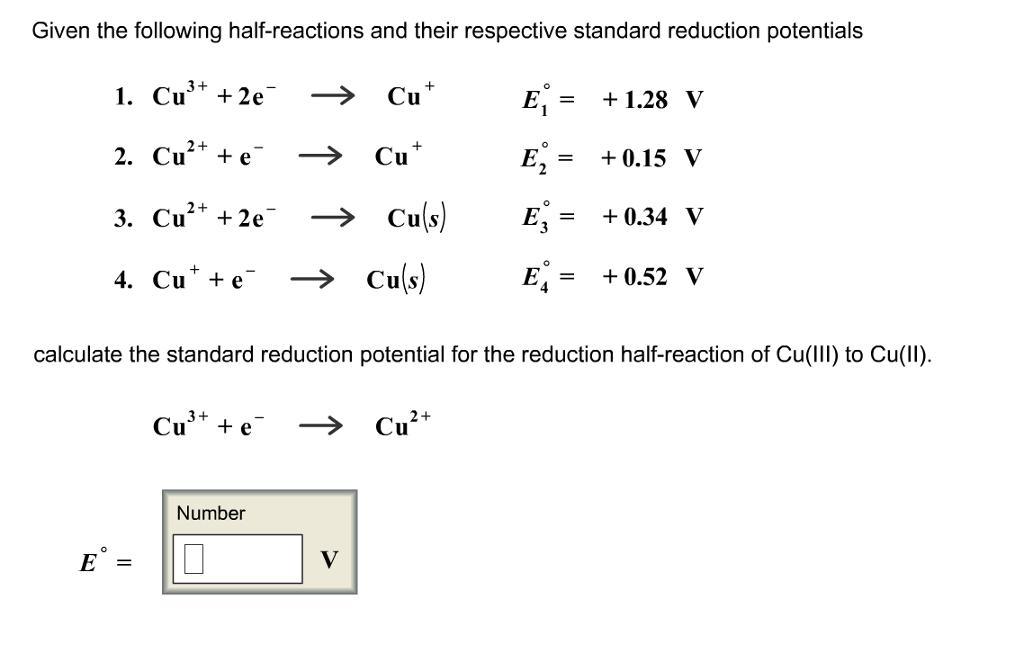
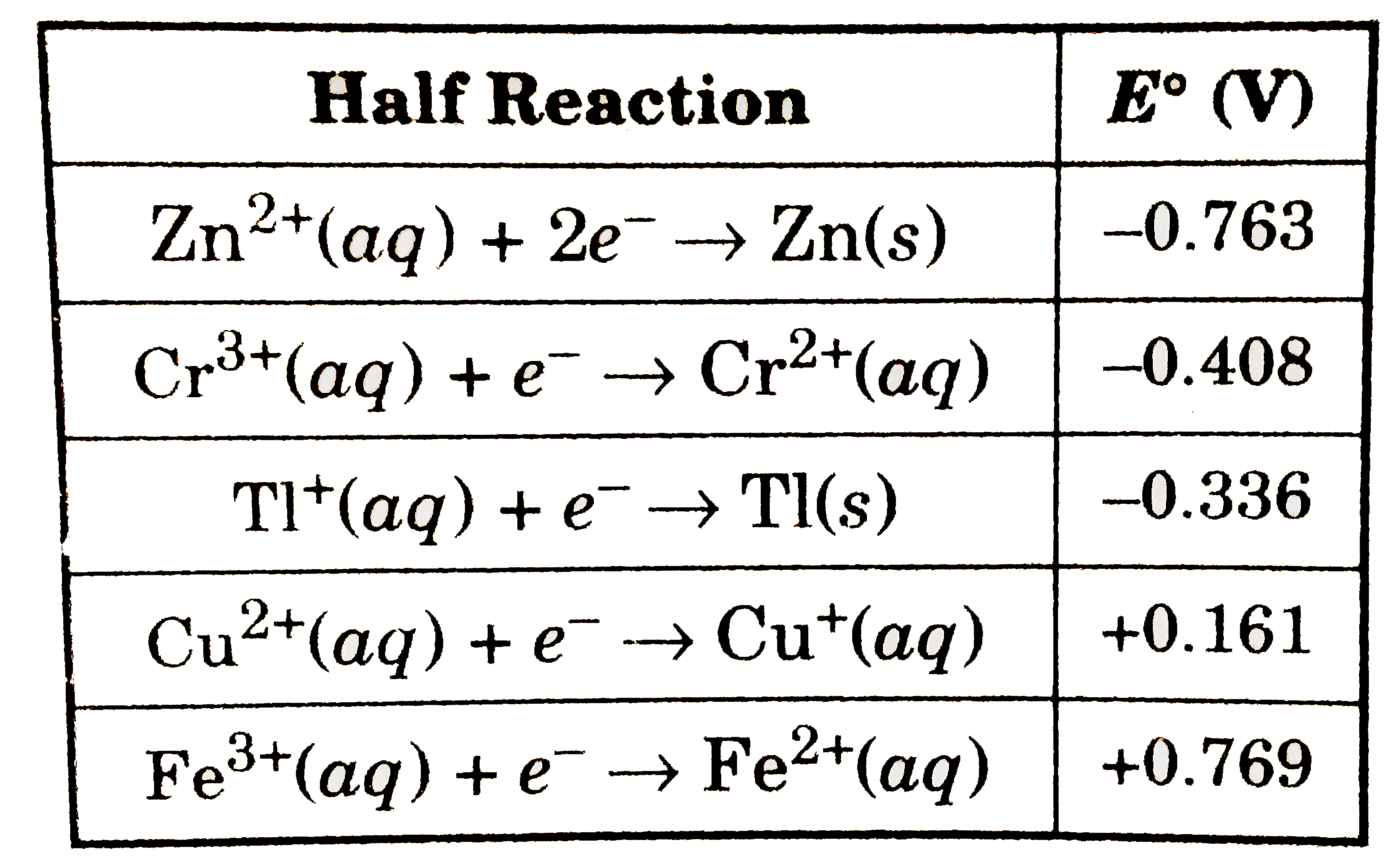
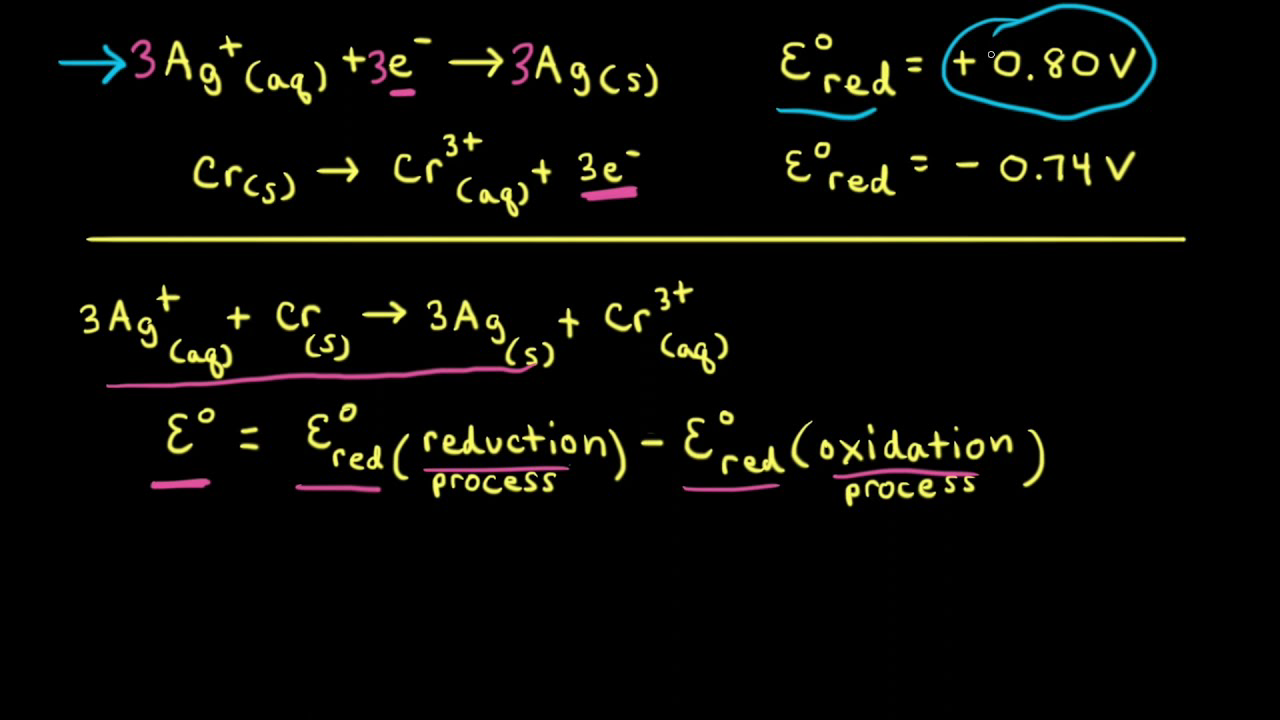The standard reduction potential for the half cell: NO3^-(aq.) + 2H^+(aq.) + e^ - → NO2(g) + H2O is 0.78 V. Calculate the reduction potential in 8M H^+ .
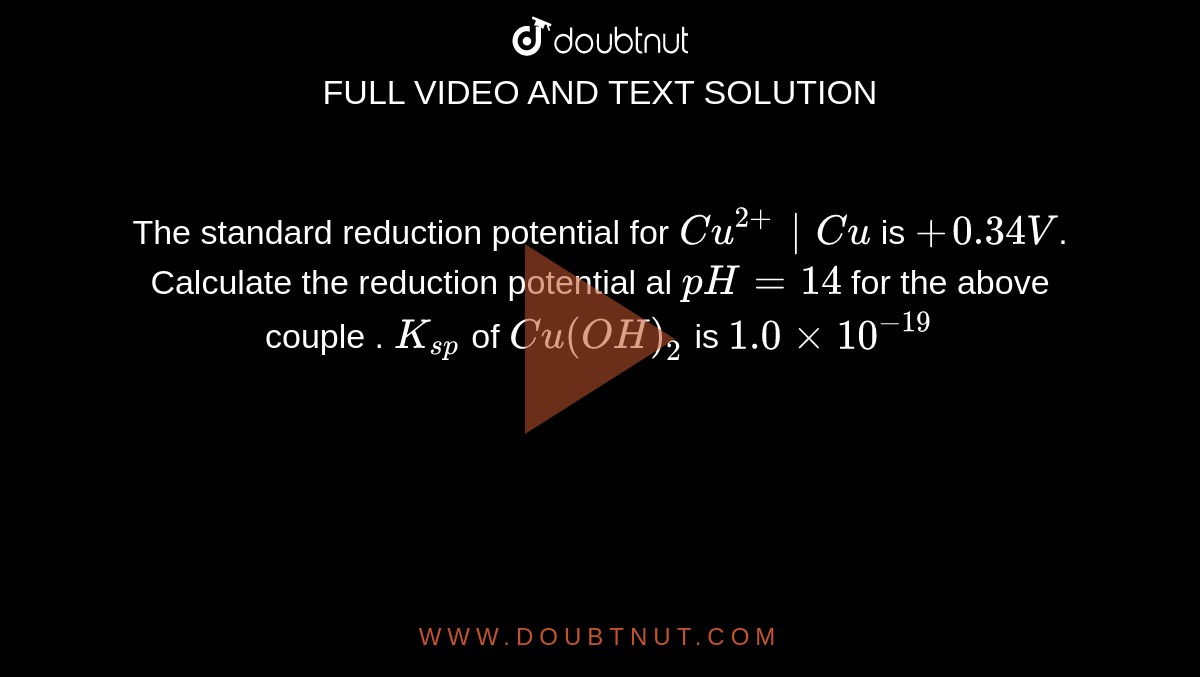
The standard reduction potential for Cu^(2+)|Cu is +0.34V. Calculate the reduction potential al pH=14 for the above couple . K(sp) of Cu(OH)(2) is 1.0xx10^(-19)

OneClass: Standard reduction potential help! Bicarbonate deprotonates in water with the formation of ...

Calculation of Standard Reduction Potentials of Amino Acid Radicals and the Effects of Water and Incorporation into Peptides. | Semantic Scholar
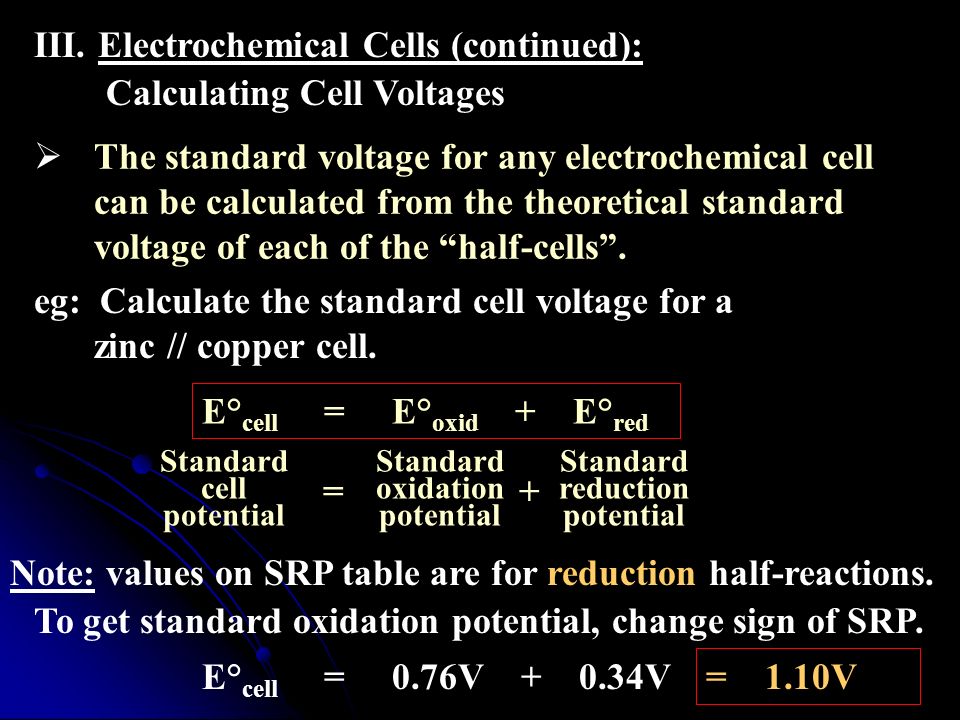
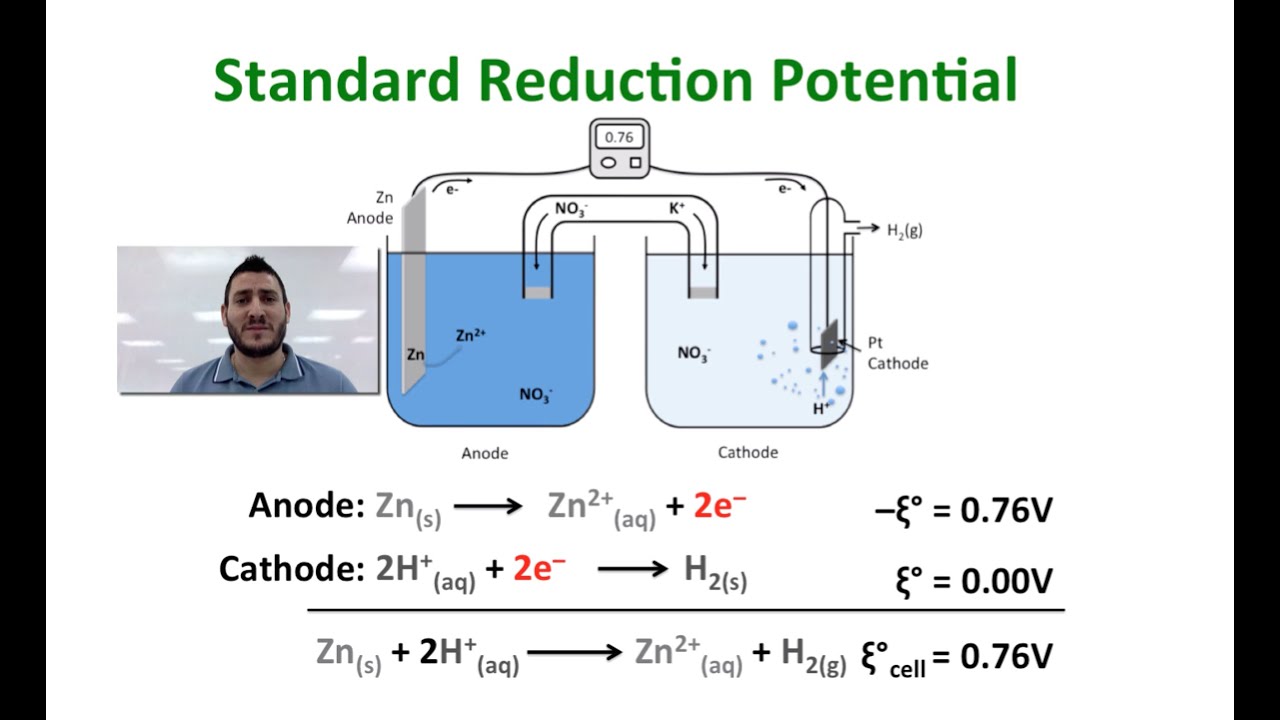
III.Electrochemical Cells (continued): Calculating Cell Voltages The standard voltage for any electrochemical cell can be calculated from the theoretical. - ppt download

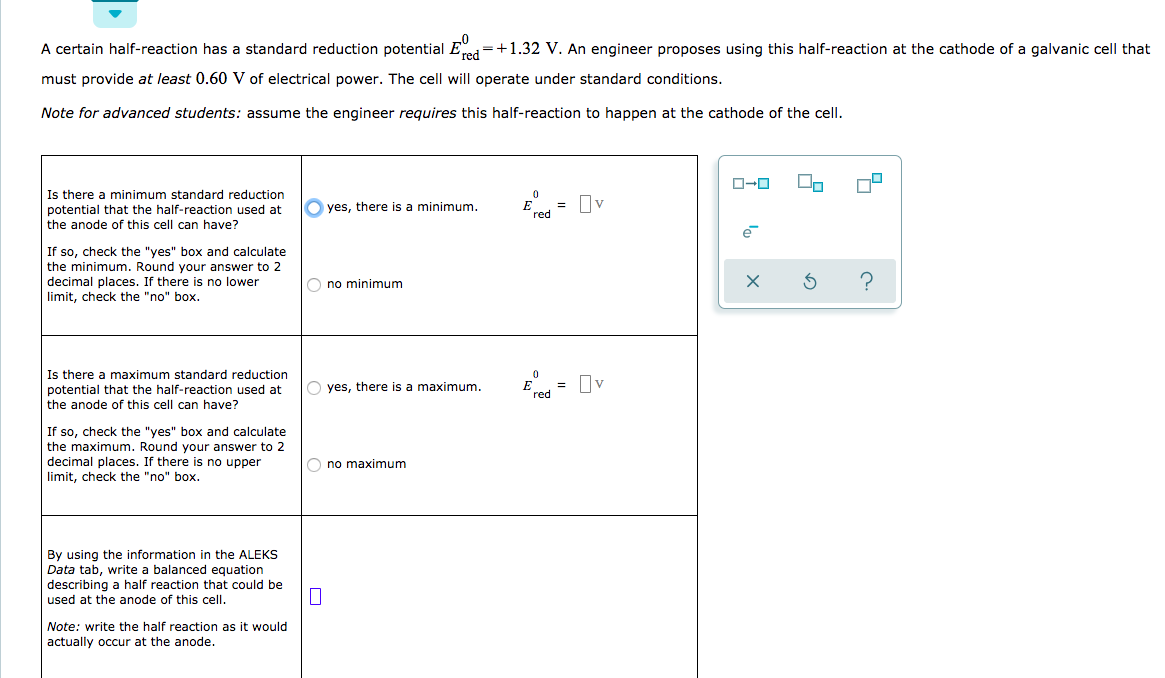
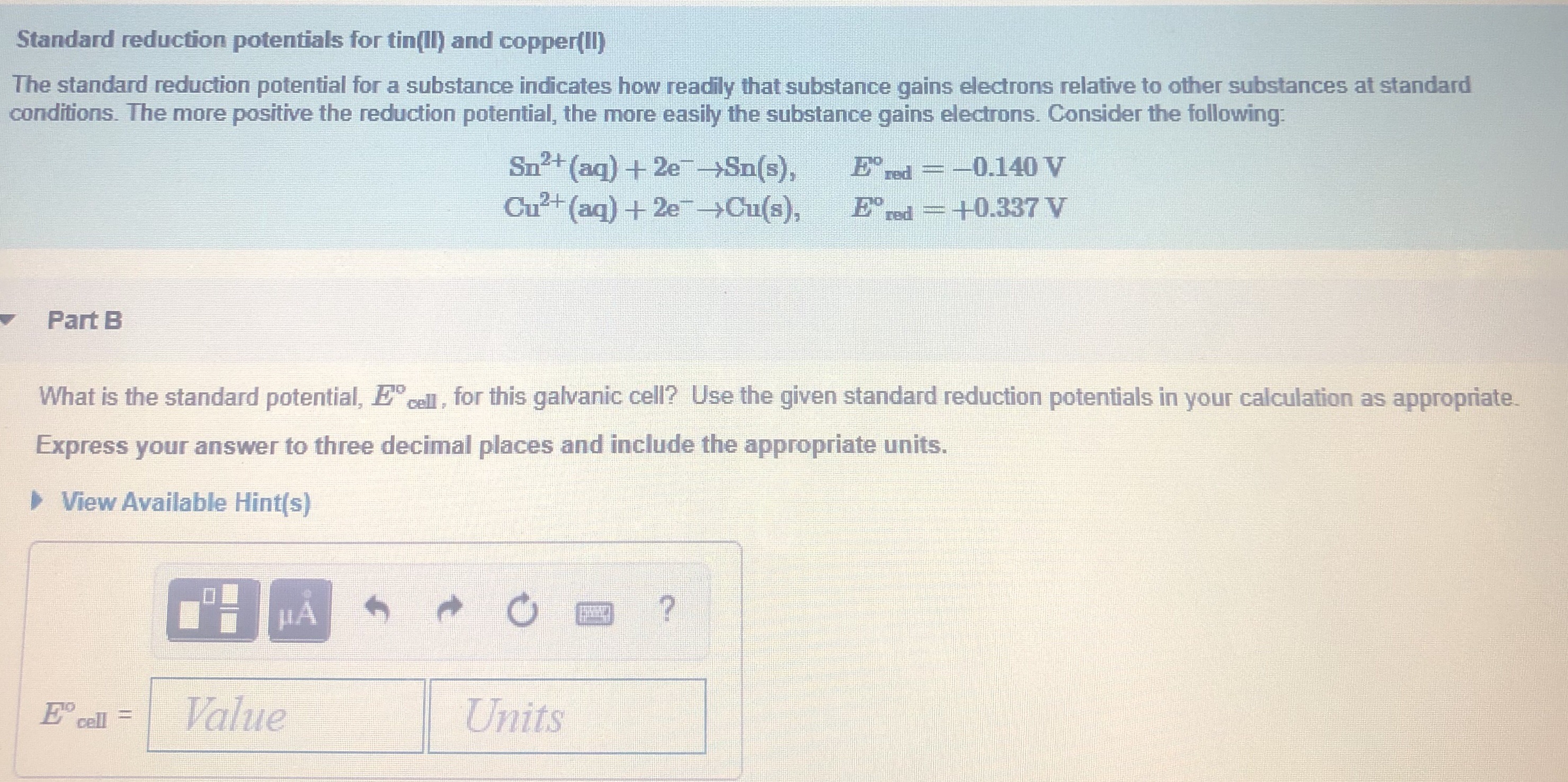
![ANSWERED] A certain half-reaction has a standard re... - Physical Chemistry ANSWERED] A certain half-reaction has a standard re... - Physical Chemistry](https://media.kunduz.com/media/sug-question/raw/59671895-1659273579.31138.jpeg)