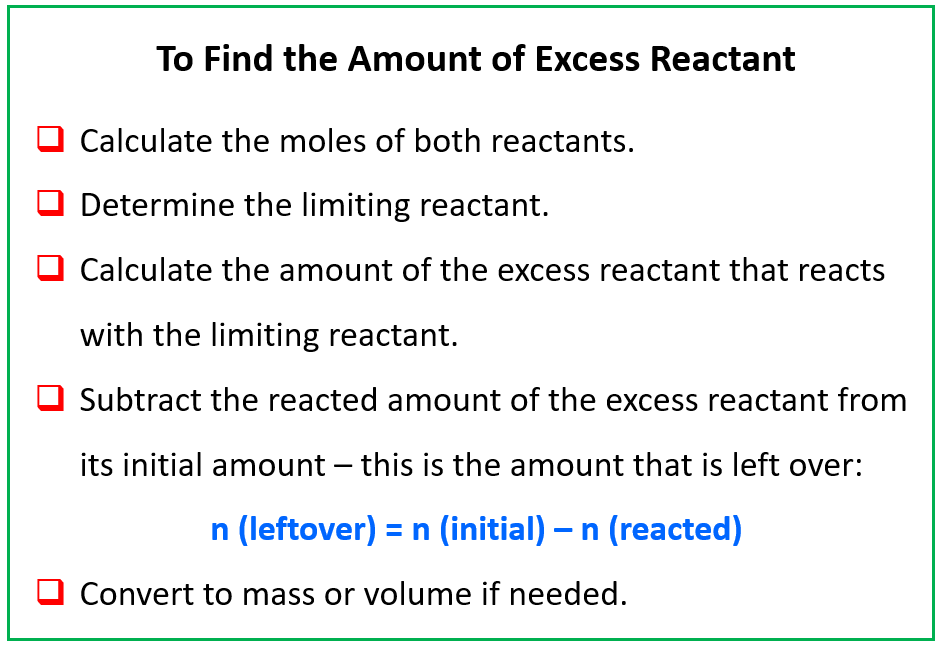
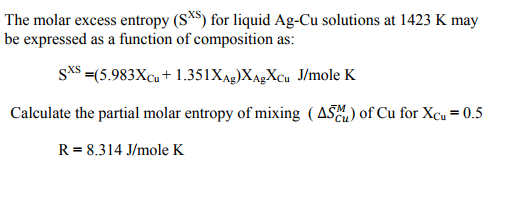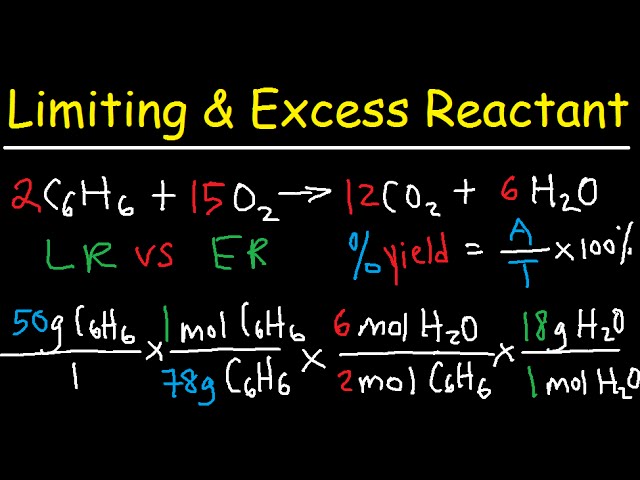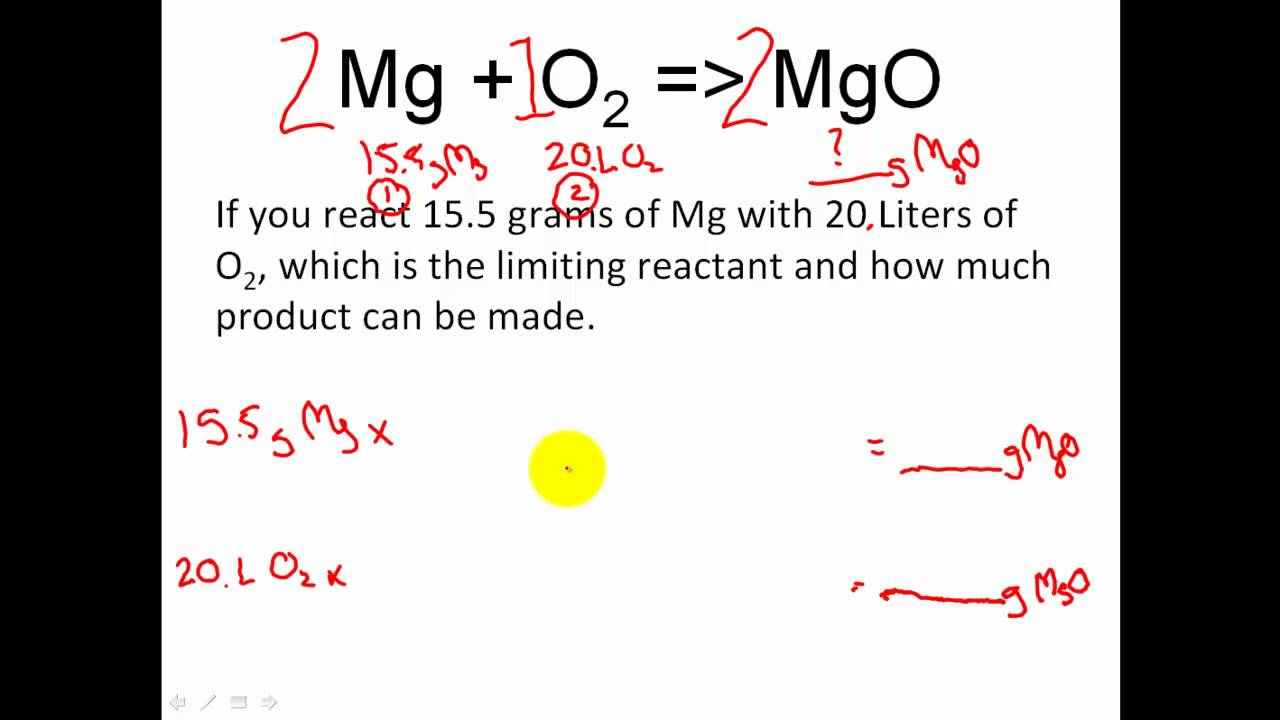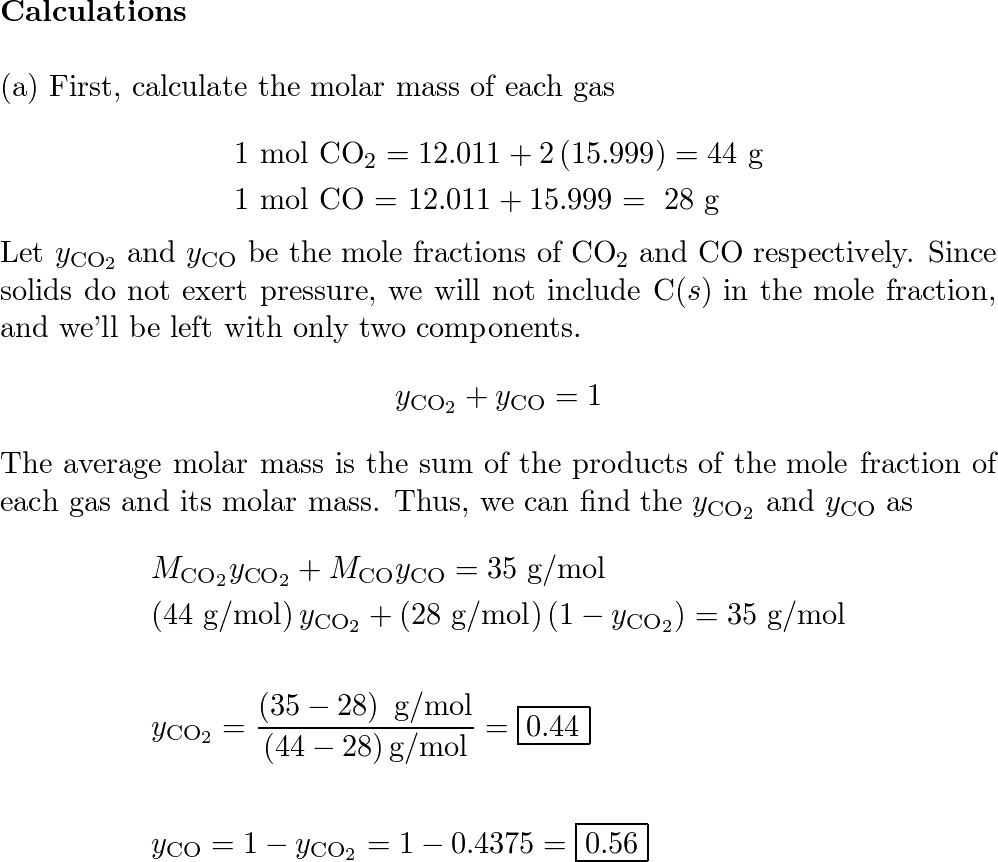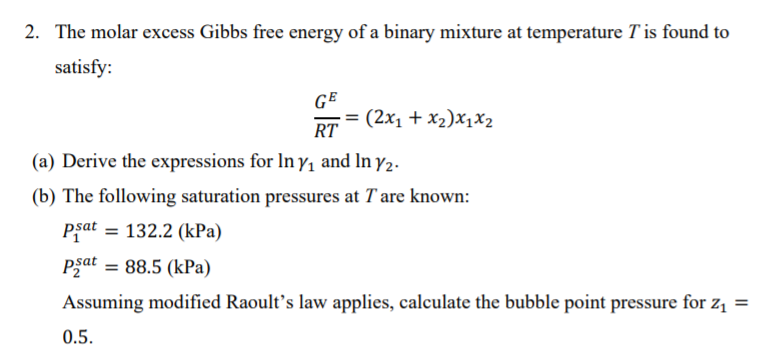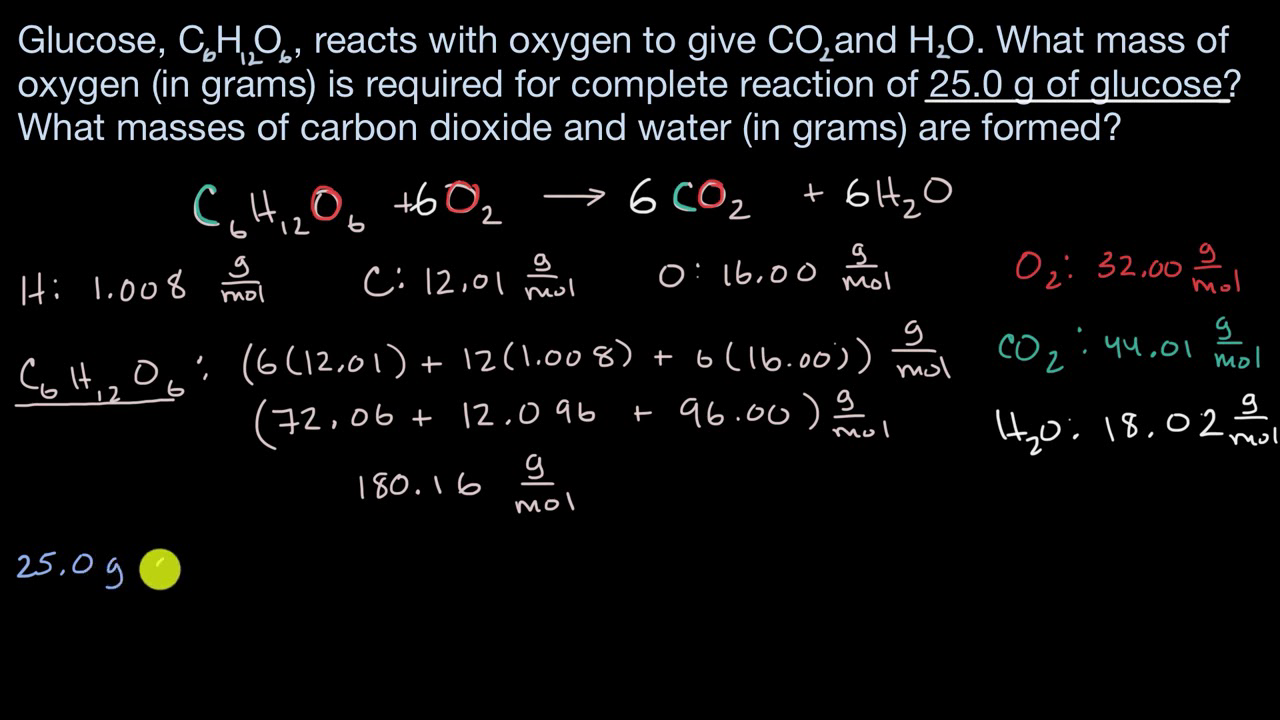SOLVED: Calculate the mass of water produced when 9.47 8 of u methane CHa reacts with an excess of oxygen in the following reaction: CH4 + 202 COz + 2Hz0 HzO molar
Theoretical, Actual, Percent Yield & Error - Limiting Reagent and Excess Reactant That Remains - YouTube

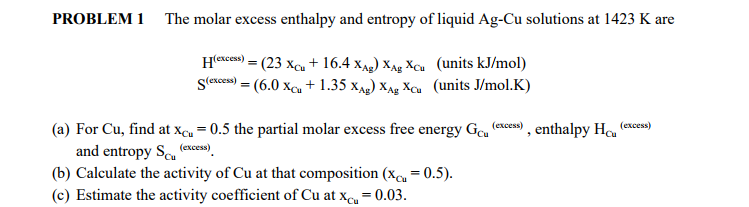
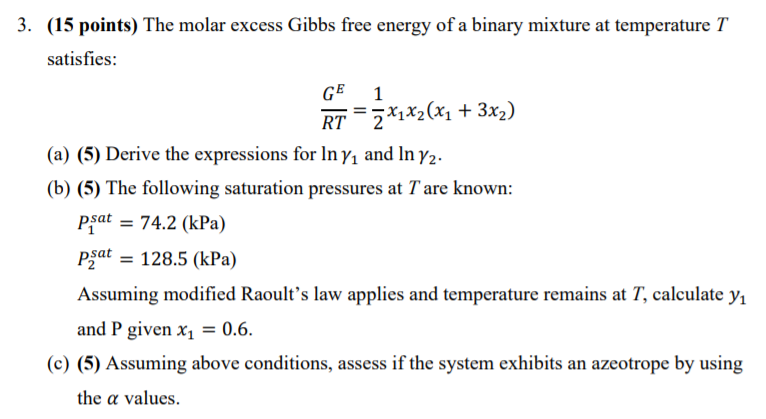
SOLVED: The molar excess Gibbs free energy of formation of solid solutions in the 'system Au-Ni can be represented by G*s '=XiXAu(QAIAOX Au + 38280X Ni 14230XAuX Ni 2660 Calculate the activities

OneClass: Acetylene gas (C2H2) is burned completely with 20 percent excess air during a steady state-...

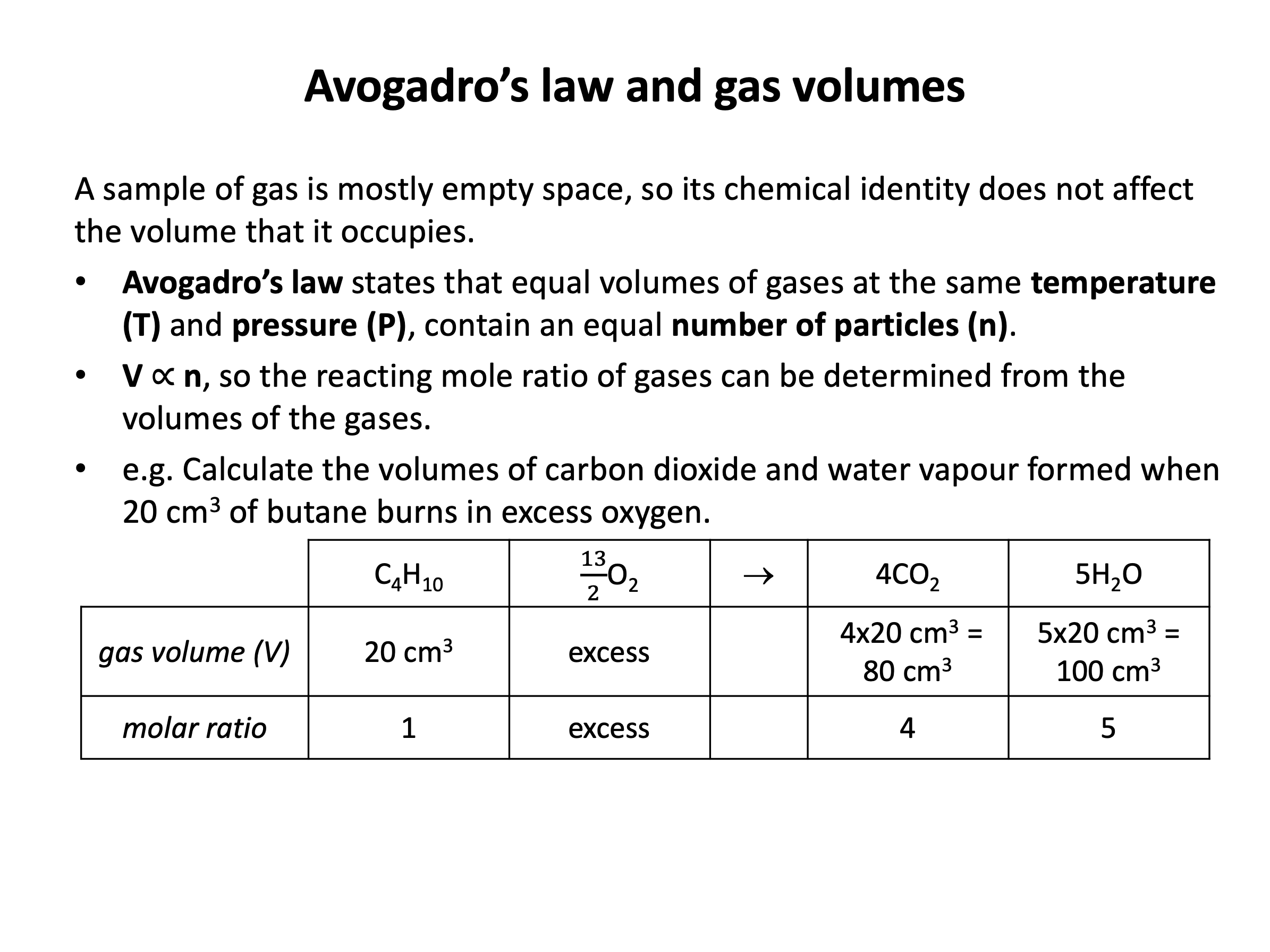
molar gas volume Avogadro's Law moles and mass calculations gcse chemistry calculations igcse KS4 science A level GCE AS A2 O Level practice questions exercises

PDF) Calculation of excess enthalpy of binary mixtures with using of excess volume experimental data

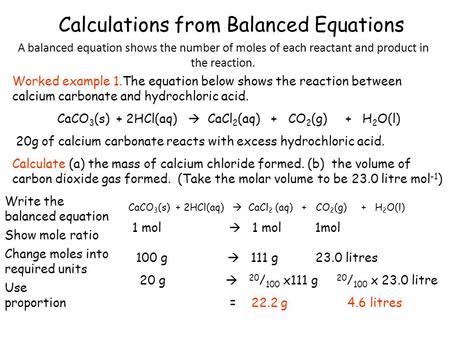
Given the equation: Cu + AgNO 3 → Ag + CuNO 3 , what mass of silver in grams is precipitated when 40.0 g of copper reacts with an excess of silver nitrate in solution?