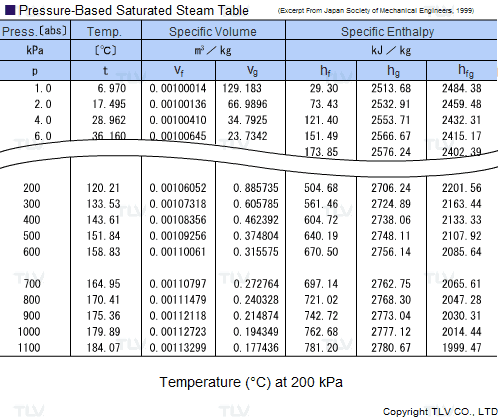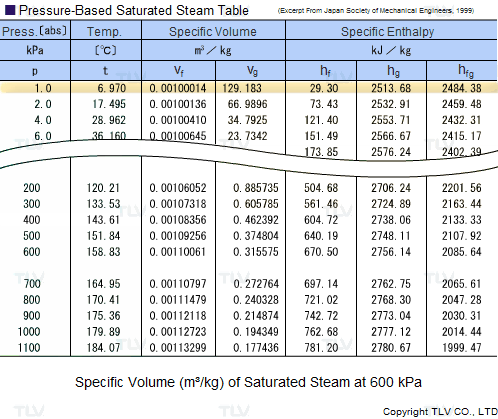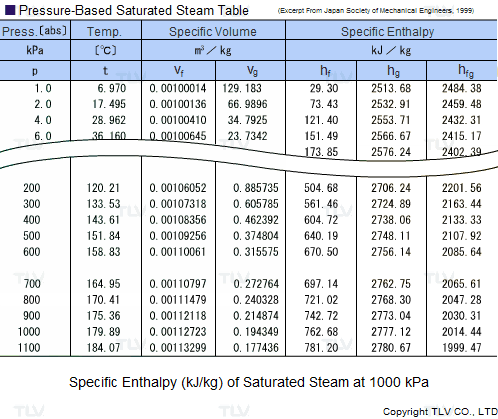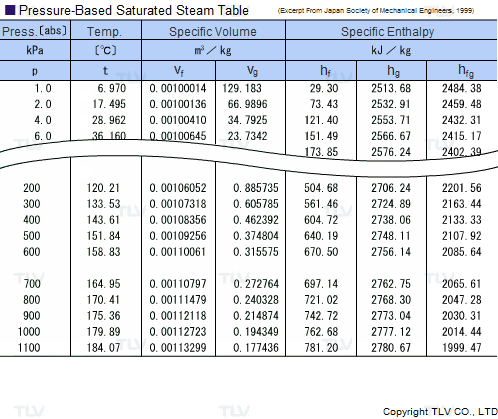
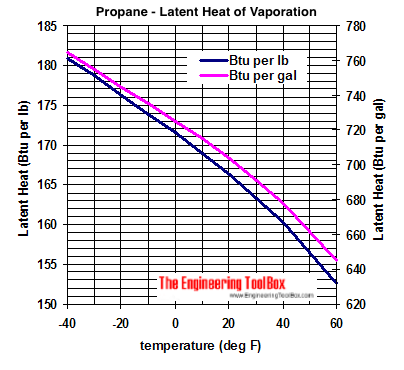
Calculate the molal elevation constant, kb for water and the boiling point of 0.1 molal urea solution. Latent heat of vaporisation of water is 9.72 kcal mol ^-1 at 373.15 K.

Calculate the entropy change for vaporization of `1mol` of liquid water to stem at `100^()C`, if... - YouTube

Using Heat of Fusion or Vaporization to Find the Heat Needed to Melt or Boil a Substance | Chemistry | Study.com
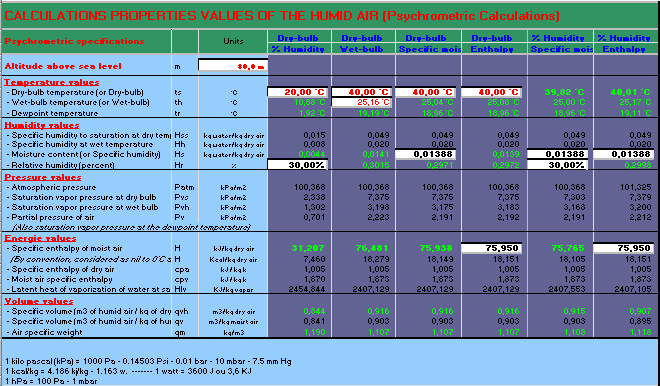
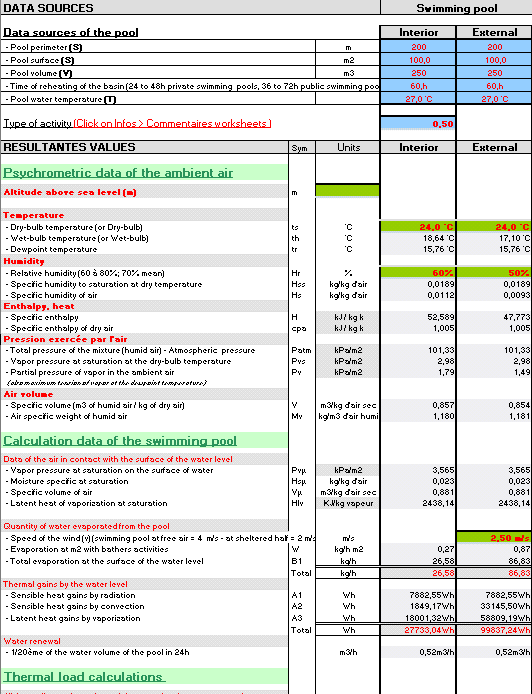
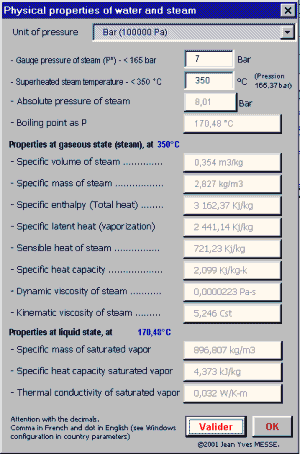
Misting, Evaporative, cooling, fogging, nozzles, temperature, humidity, moiture, dew, excel, calculation

Calculate the enthalpy of vaporisation per mole for ethanol. Given, Δ S = 109.8JK^-1mol^-1 and boiling point of ethanol is 78.5^oC .

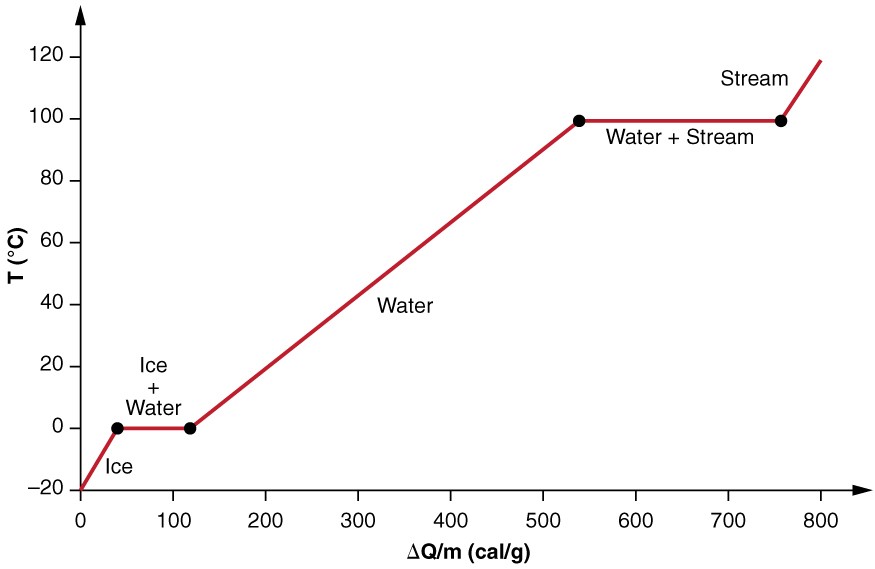
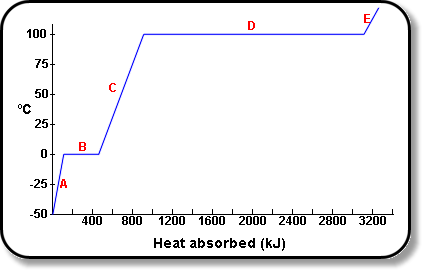
Calculate the heat required to convert 3 kg of ice at - 12^o C kept in a calorimeter to steam at 100^o at atmospheric pressure. (Given: specific heat of ice = 2.100 ×
![Using some or all of the information below, calculate the standard molar entropy of I2 at 450 K. S^o = [{Blank}] J/K.mol at 450 K. | Homework.Study.com Using some or all of the information below, calculate the standard molar entropy of I2 at 450 K. S^o = [{Blank}] J/K.mol at 450 K. | Homework.Study.com](https://homework.study.com/cimages/multimages/16/screen_shot_2020-12-02_at_3.01.47_am7814899012014415578.png)