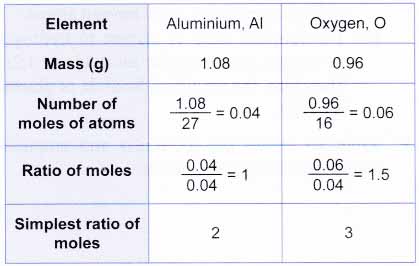
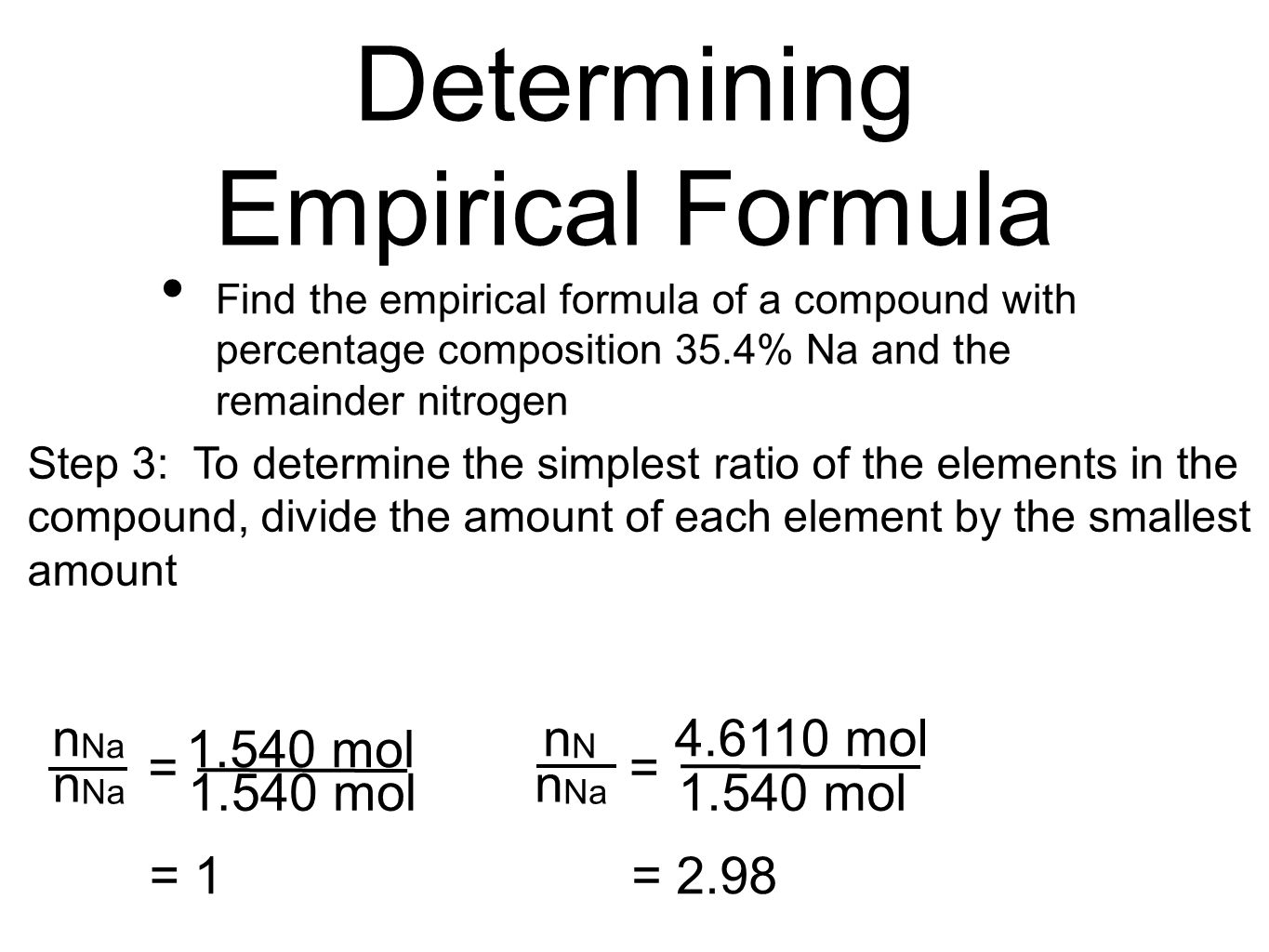
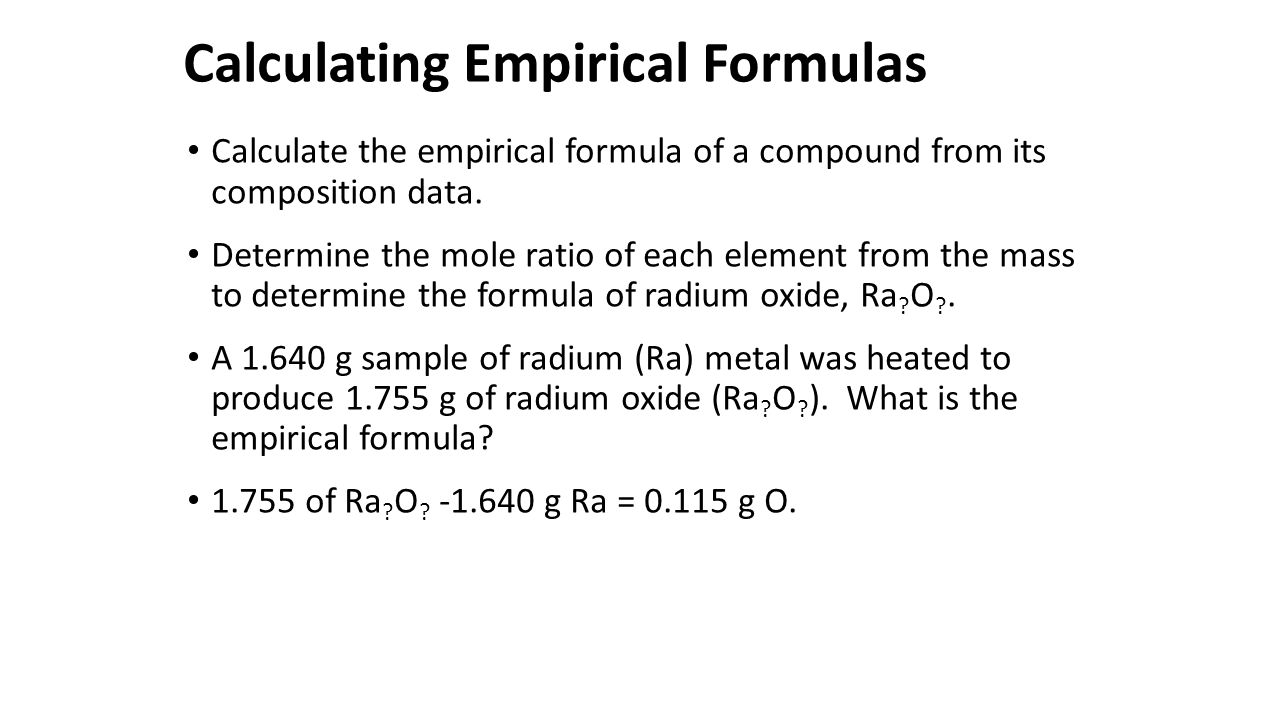
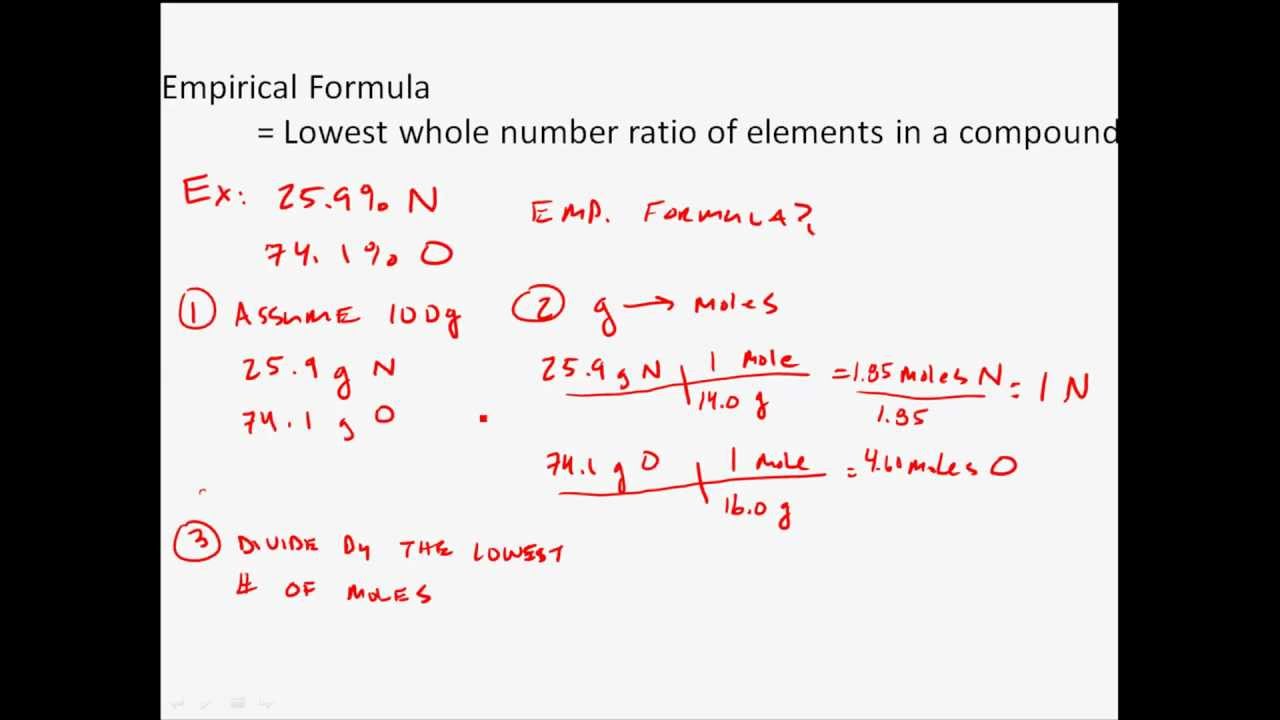
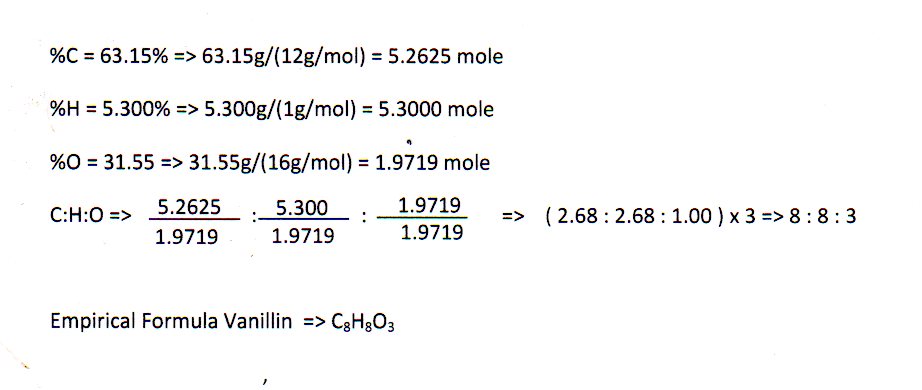
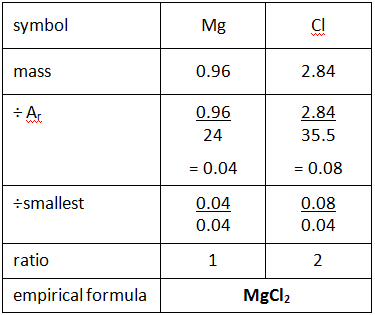
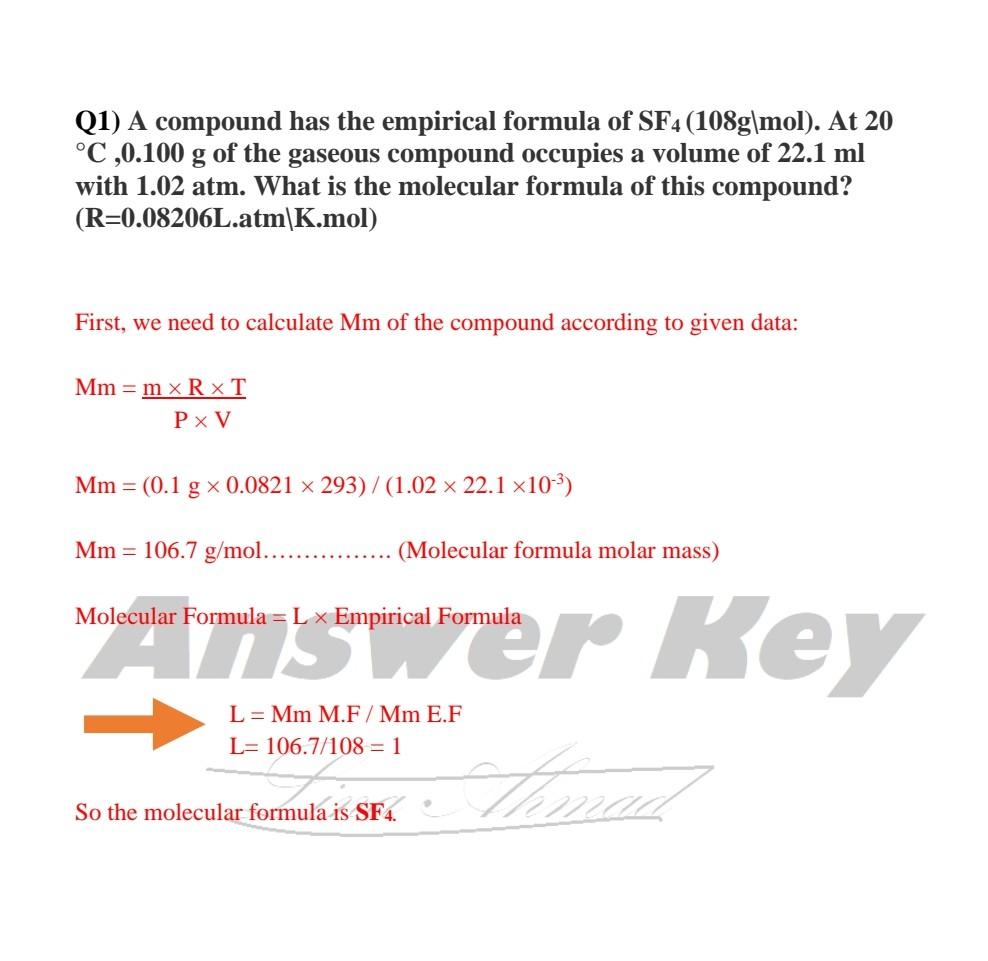
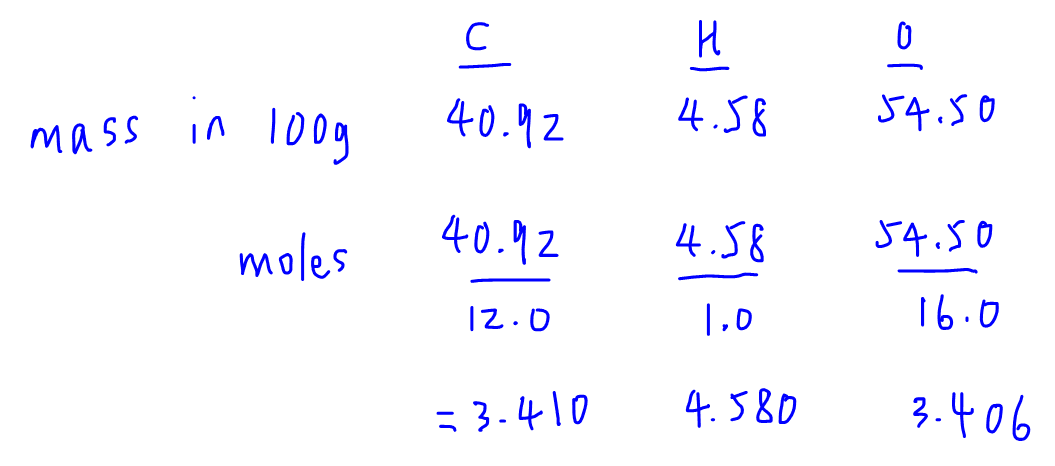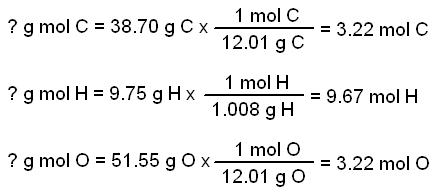The empirical formula of the compound is CHO2 . The molecular weight of the compound is 90. Calculate the molecular formula of the compound.

Calculate the Empirical Formula for a compound with the following composition: 46.16% carbon; 53.84% nitrogen 1)Change % to grams (if needed) 2)Convert. - ppt download

Calculate the Empirical Formula of a compound whose percentage composition is C=21 9% , H=4 6% and Br=73 4% - Chemistry - - 7857347 | Meritnation.com
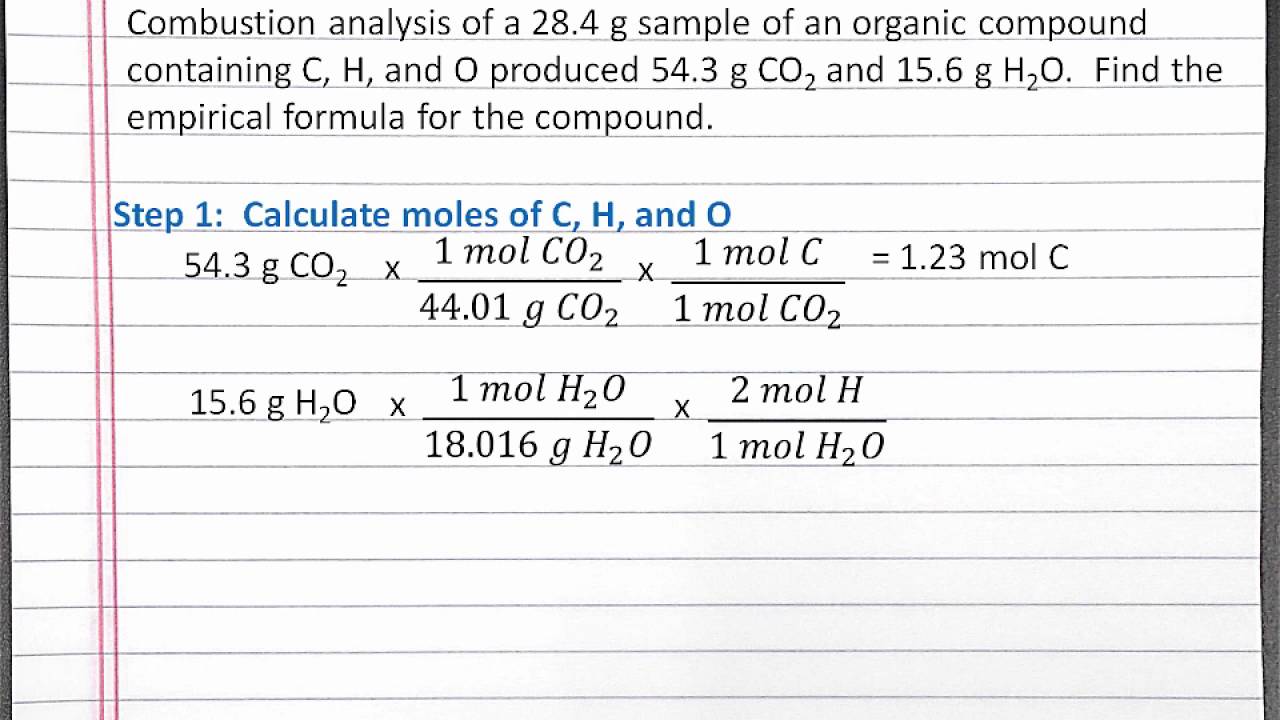
CHEMISTRY 101: Finding Empirical Formula Using Combustion Analysis for a Compound with C, H, O - YouTube