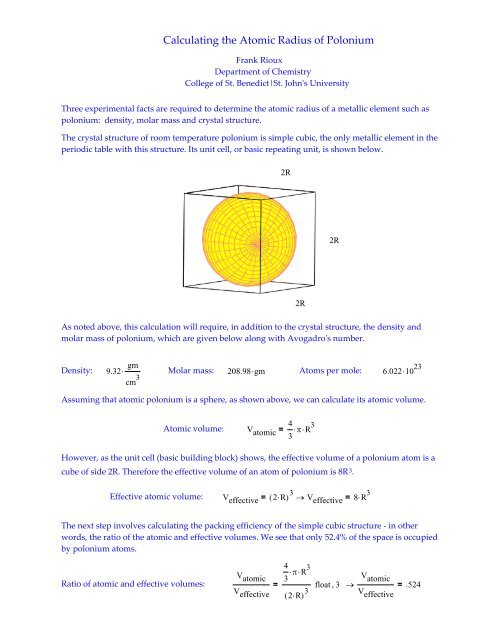
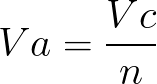What is Atomic Packing Factor (and How to Calculate it for SC, BCC, FCC, and HCP)? – Materials Science & Engineering
Welcome to Chem Zipper.com......: How to calculate percentage (%) ionic character in covalent compounds?
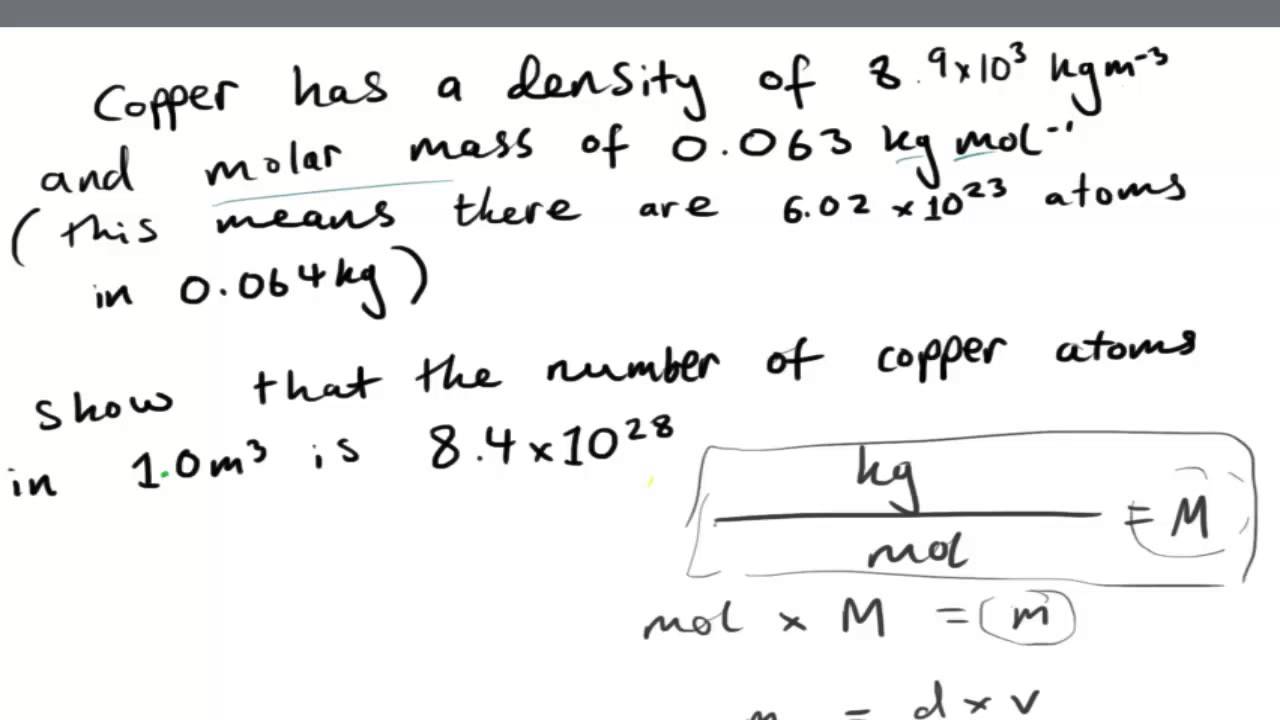
Working out the Number of Atoms in a given volume of Copper from Density/Molar Mass- AS Physics - YouTube

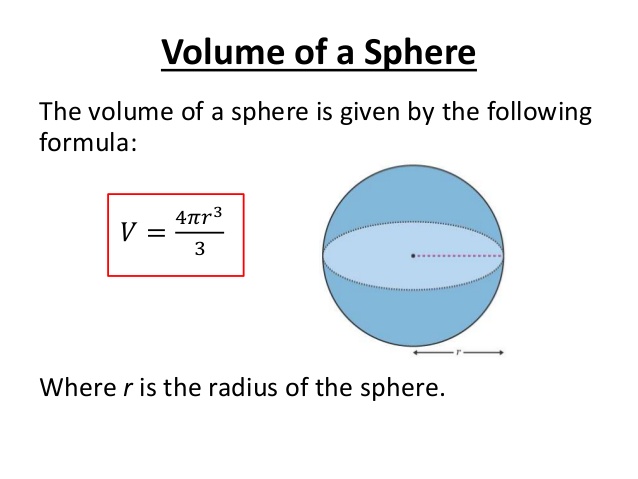
A metal M of atomic weight 54.9 has a density of 7.42 g cm^(-3). Calculate the volume occupied and the radius of the atom of this metal assuming it to be sphere.

molar gas volume Avogadro's Law moles and mass calculations gcse chemistry calculations igcse KS4 science A level GCE AS A2 O Level practice questions exercises
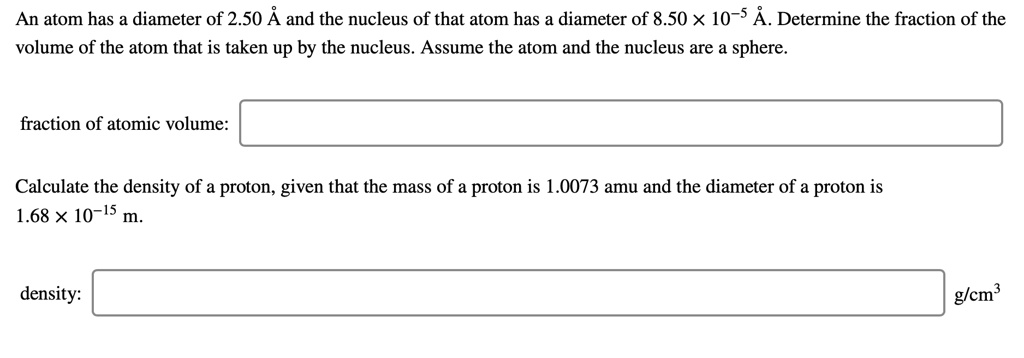
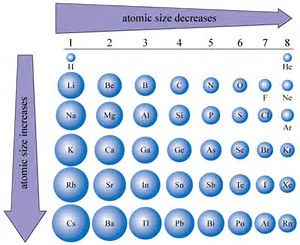
SOLVED: An atom has diameter of 2.50 A and the nucleus of that atom has diameter of 8.50 X 10-5 A. Determine the fraction of the volume of the atom that is