
Which solvent, water or carbon tetrachloride, would you choose to dissolve each of the following? a. KrF_2 b. SF_2 c. SO_2 d. CO_2 e. MgF_2 f. CH_2O g. CH_2=CH_2 | Homework.Study.com

SOLVED: Consider two solvents that are commonly used for organic chemistry reactions: CH2Cl2 and CCl4. Interestingly, studies have shown that one of these solvents is polar and one is non polar. Draw
Carbon Tetrachloride Molecule Carbon Dioxide Chemical Polarity, PNG, 1100x1062px, Carbon Tetrachloride, Carbon, Carbon Dioxide, Carbon Disulfide,
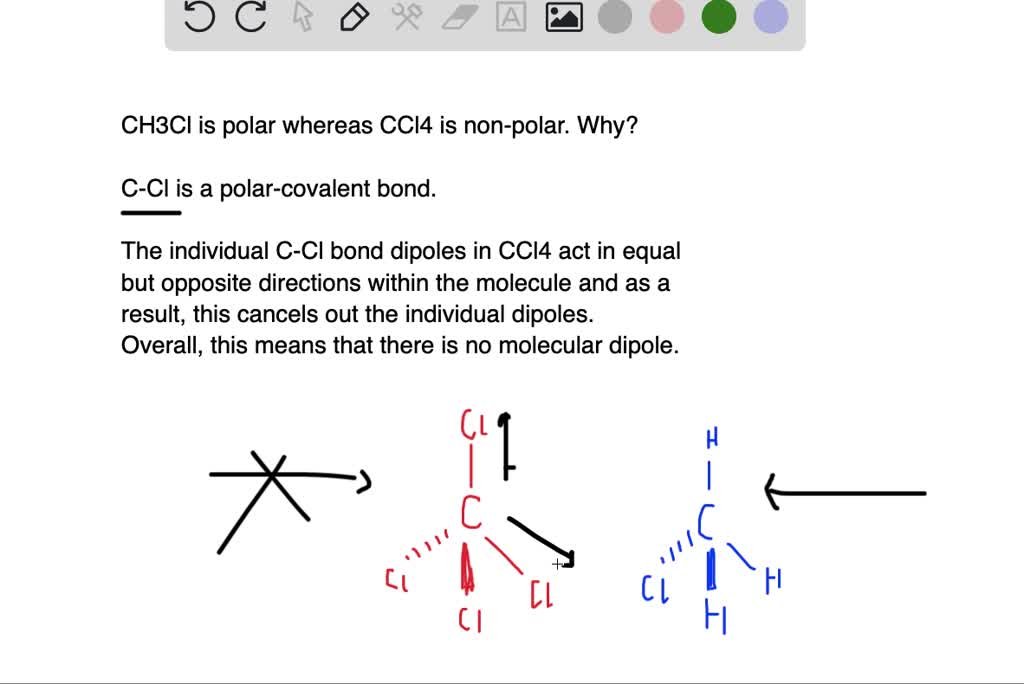
SOLVED:Account for the fact that chloromethane, CH3 Cl, which has only one polar C-Cl bond, is a polar molecule, but carbon tetrachloride, CCl4, which has four polar C-Cl bonds, is a nonpolar
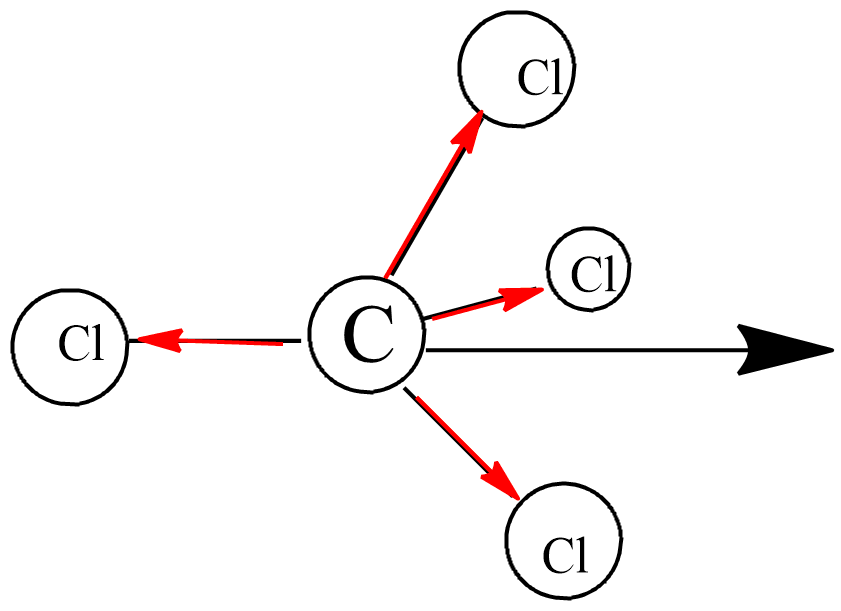

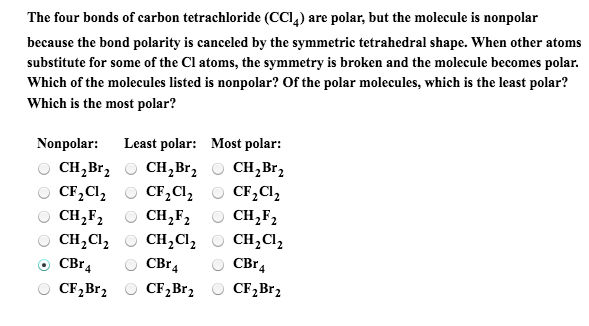







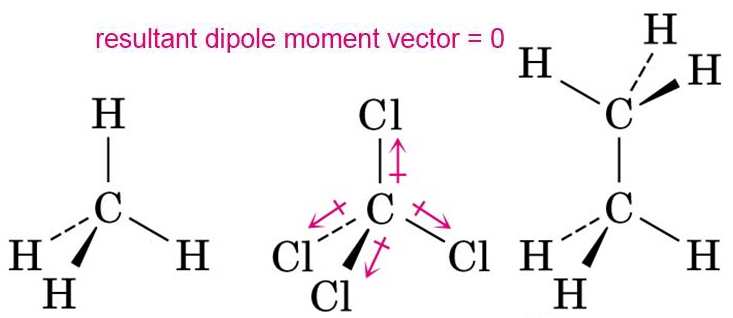




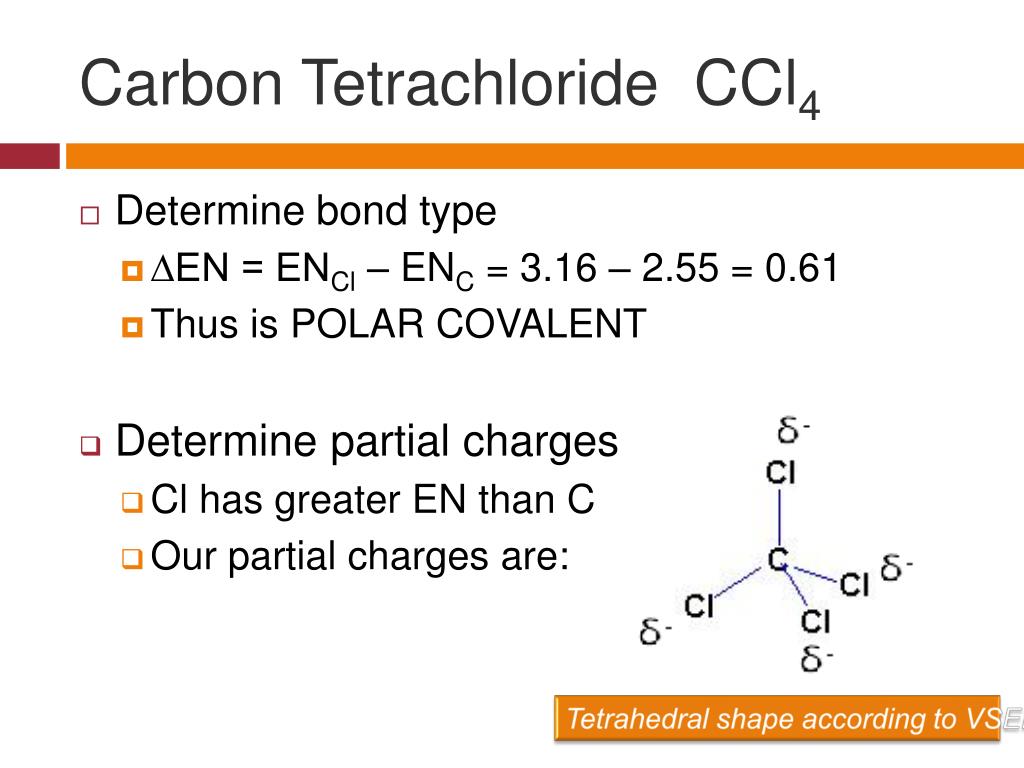

![Is \\[CC{l_4}\\] polar or nonpolar? Is \\[CC{l_4}\\] polar or nonpolar?](https://www.vedantu.com/question-sets/dbf3f5ee-35e7-43b7-b8a3-cfe63dfd520e2097468885289678636.png)