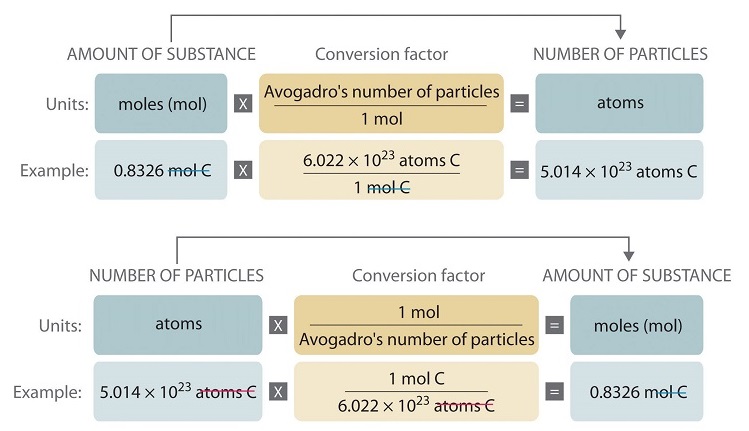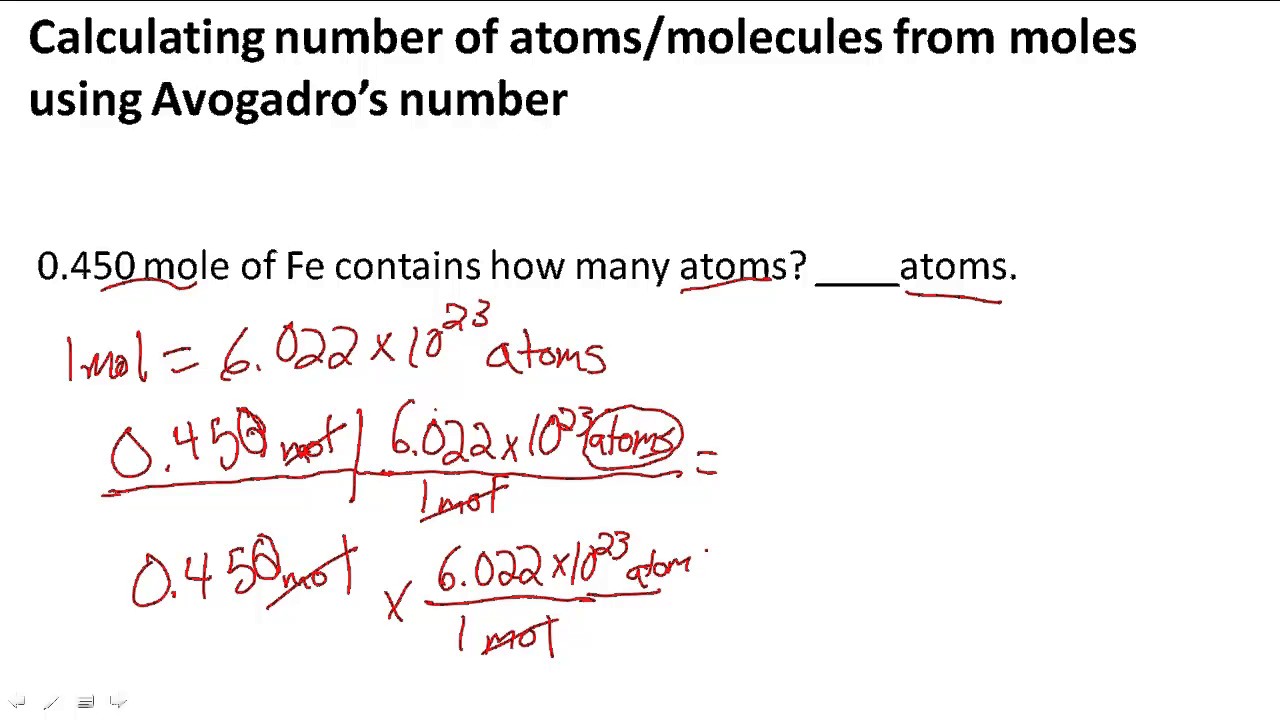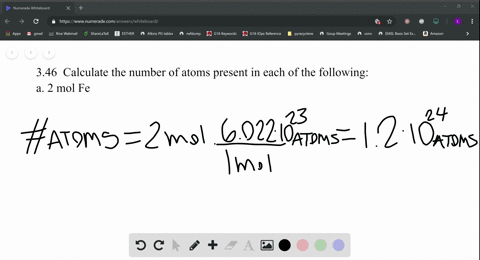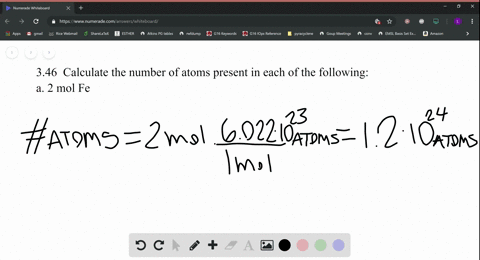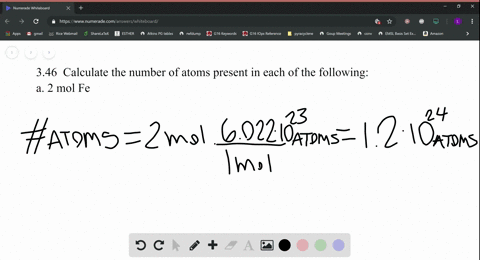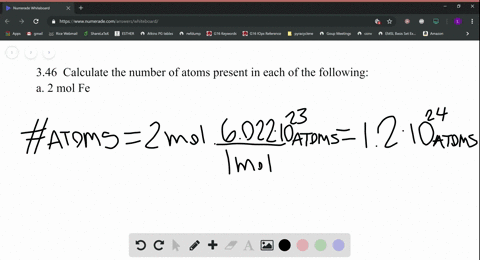
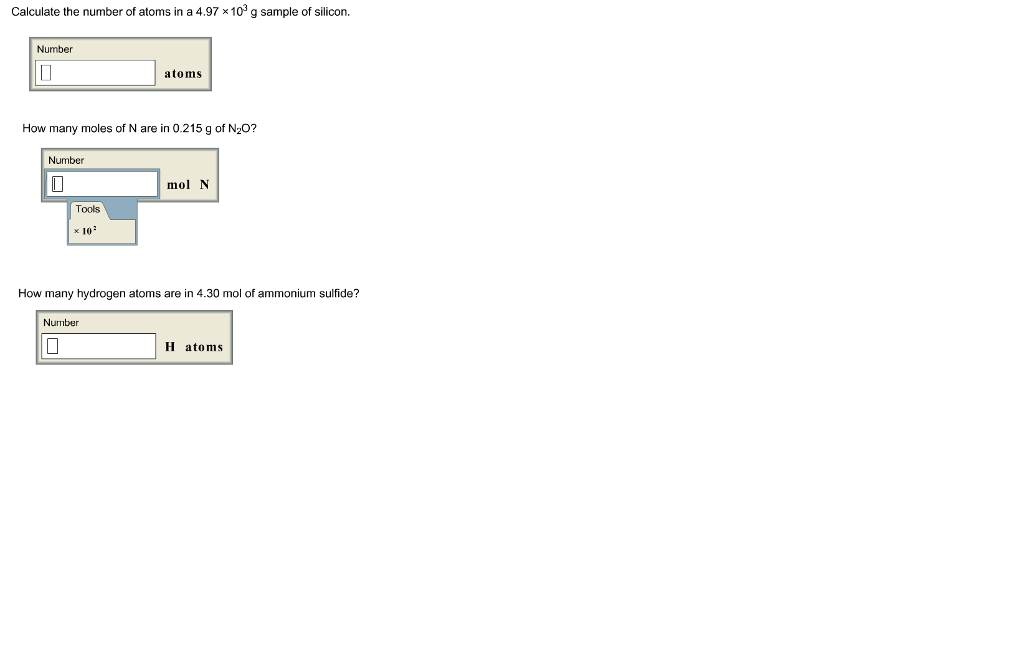
SOLVED:Calculate the number of atoms present in each of the following: a. 2 mol Fe b. 40.1 g Ca, which has an atomic mass of 40.08 amu c. 4.5 mol of boron-11

Determining the Relative Number of Atoms in a Substance using Elemental Analysis | Chemistry | Study.com

Calculate the number of atoms of oxygen present in 88 g of CO2 . What would be the weight of CO having the same number of oxygen atoms?
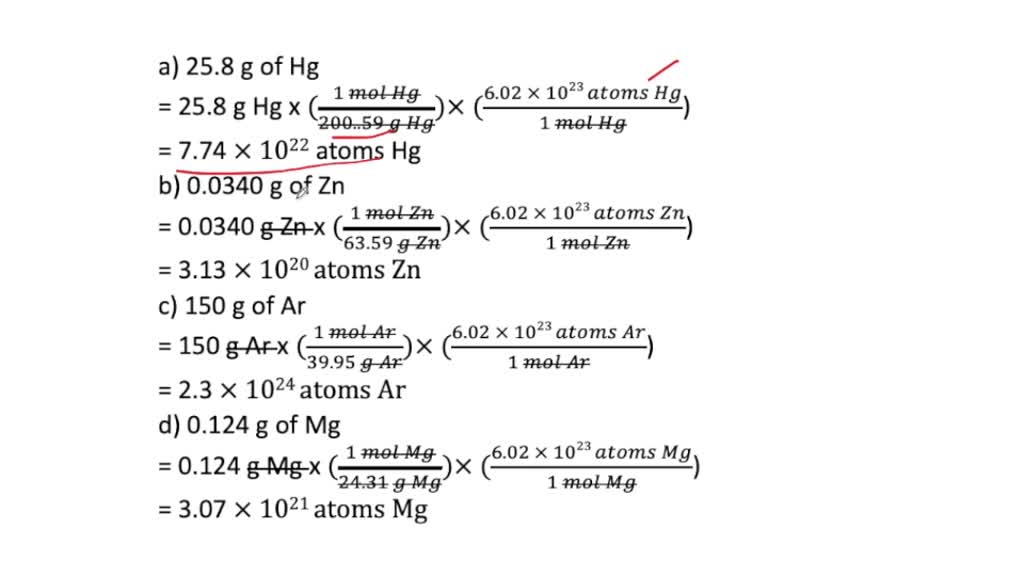
SOLVED:Calculate the number of atoms in each element. a. 25.8 g of Hg b. 0.0340 g of Zn c. 150 g of Ar d. 0.124 g of Mg
calculate number of atoms present in 11.2 L ammonia at 273 degrees celsius and 2 atm. a:6.023*10^(23) b:2*6.023*10^(23) c:0.5*6.023*10^(23)

Calculate the no.of atoms in 18g of water(H20). Please show the formula for calculating the no.of - Brainly.in
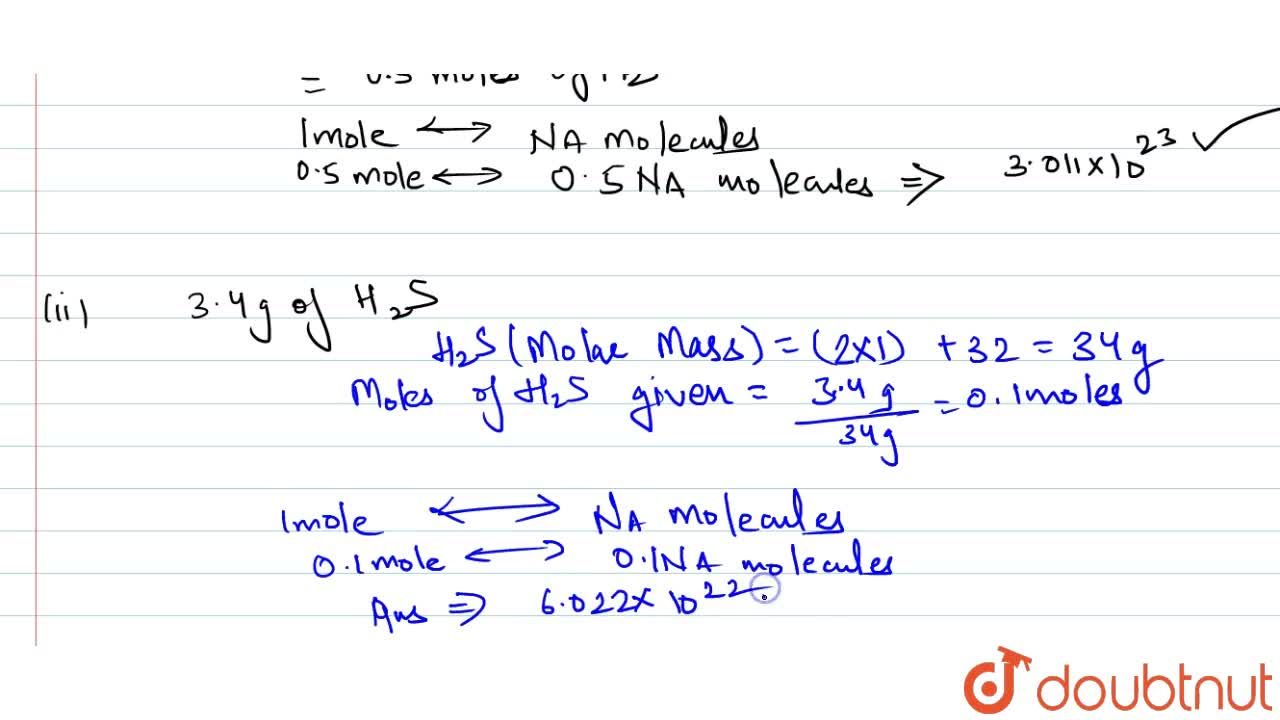
Calculate number of atoms in each of the following" i. 0.5 mol atom of nitorgen ii. 0.2 mol molecules of hydrogen iii. 3.2 g of sulphur Calculate number of molecules in each
Calculate the number of particles in each of the following: (a) 48 g of Mg (b) 8 g of O2 (c) 0.1 mole of carbon (Atomic mass Mg = 24 u, O =

Calculate the number of atoms in (i) 0.5 mole atoms of carbon (C^12) (ii) 3.2 g of sulphur (iii) 18.0 g of glucose (C6 H12O6) (iv) 0.20 mole molecules of oxygen




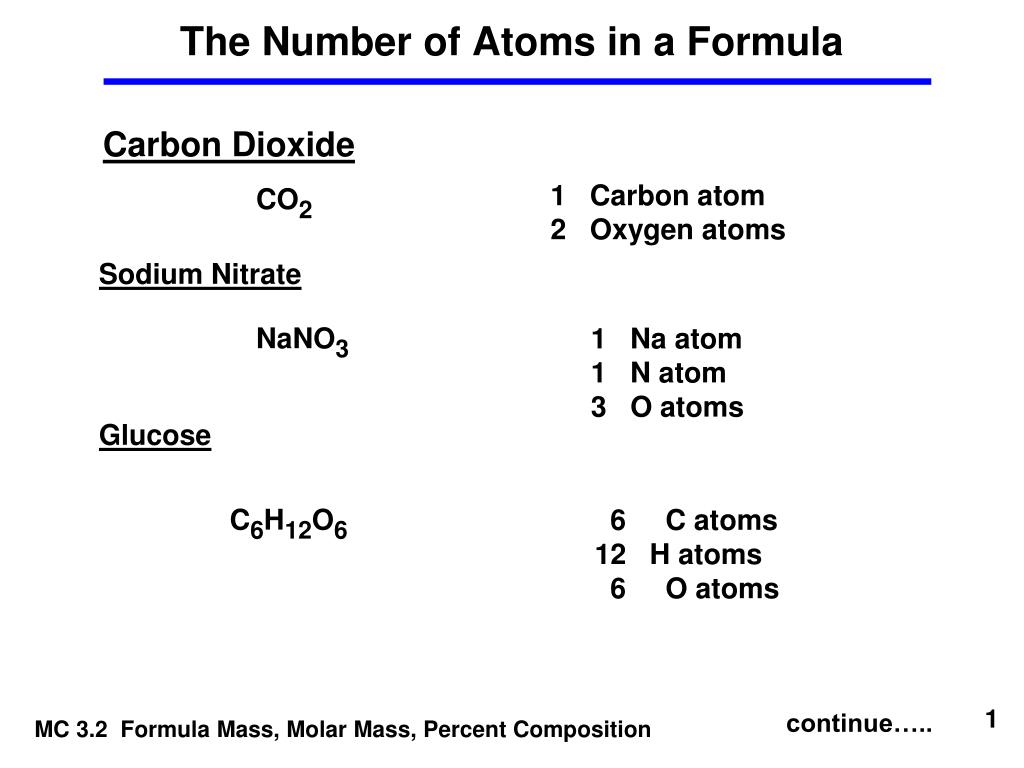


![Calculate no of atoms of each type in 1.8g glucose [Give ans in terms of NA ] Calculate no of atoms of each type in 1.8g glucose [Give ans in terms of NA ]](https://haygot.s3.amazonaws.com/questions/1294799_1324580_ans_2473354570594c17b649aed6ddb7fffd.jpg)