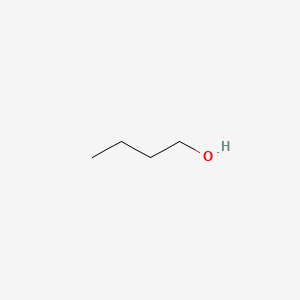
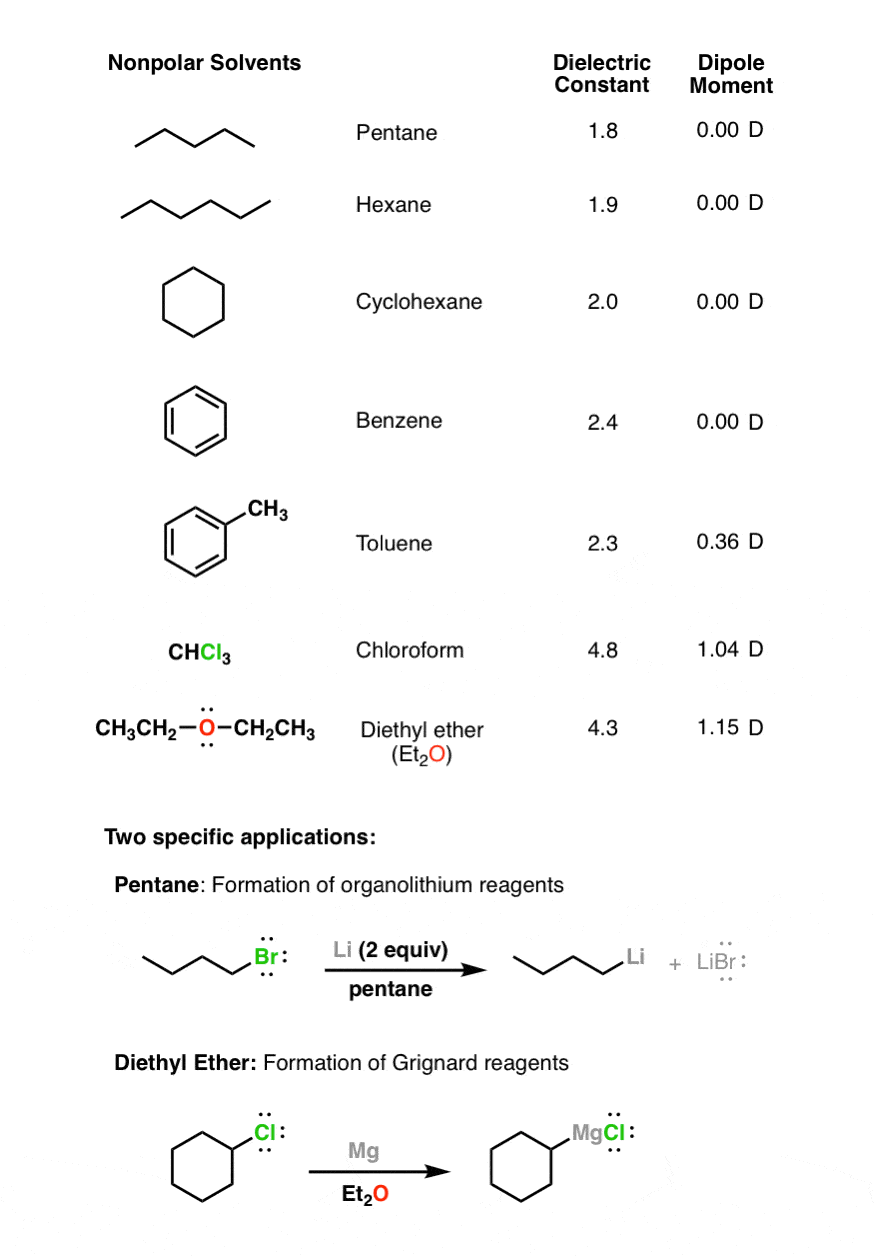
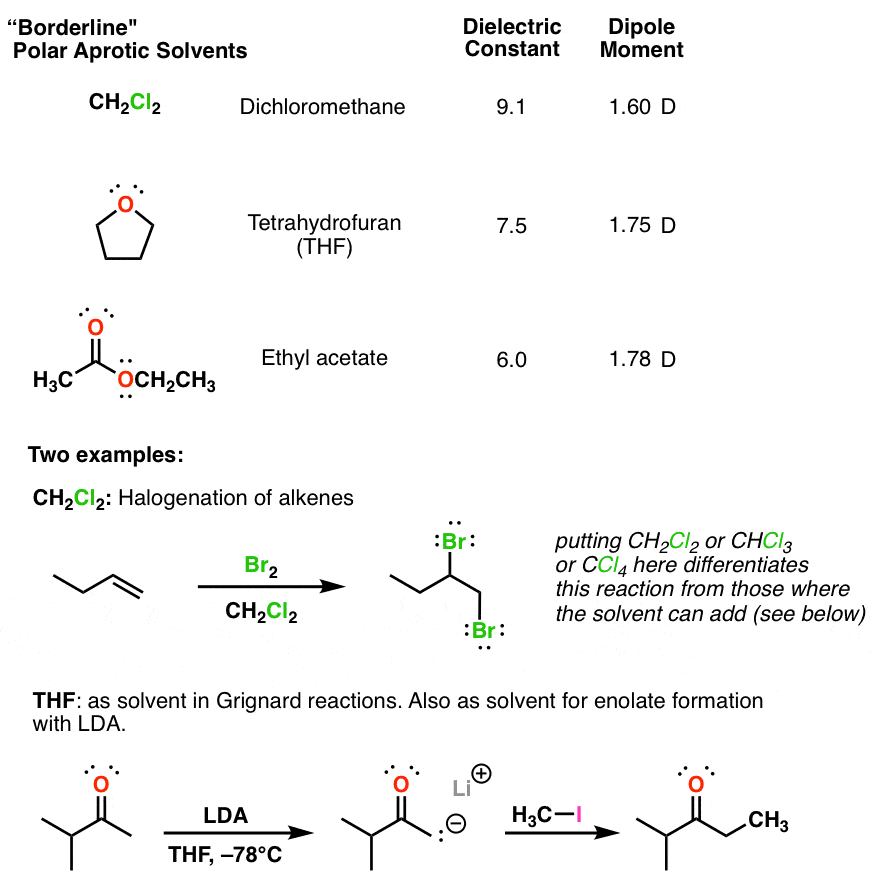
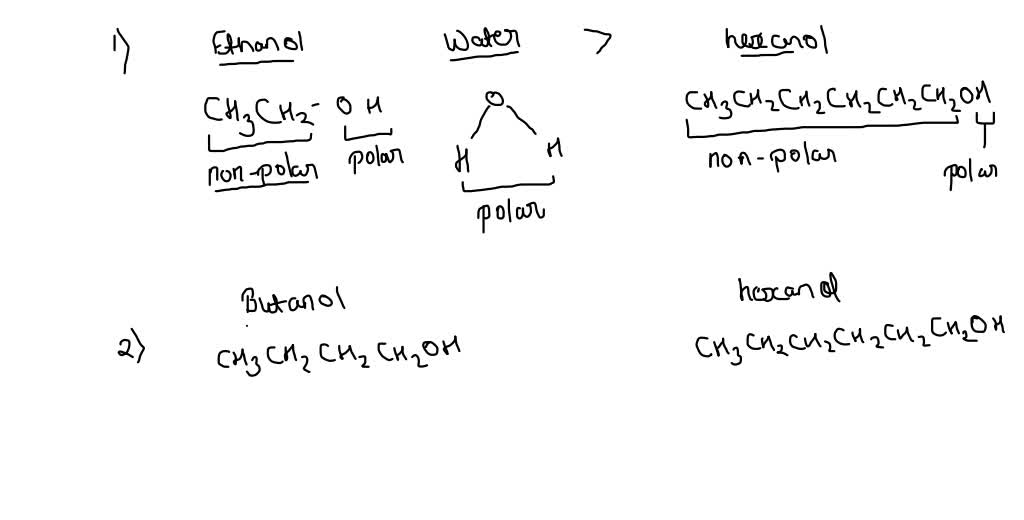
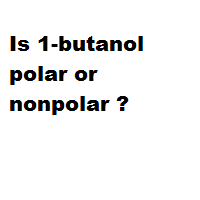Synthesis of 1-Butanol from Ethanol over Calcium Ethoxide: Experimental and Density Functional Theory Simulation | The Journal of Physical Chemistry C
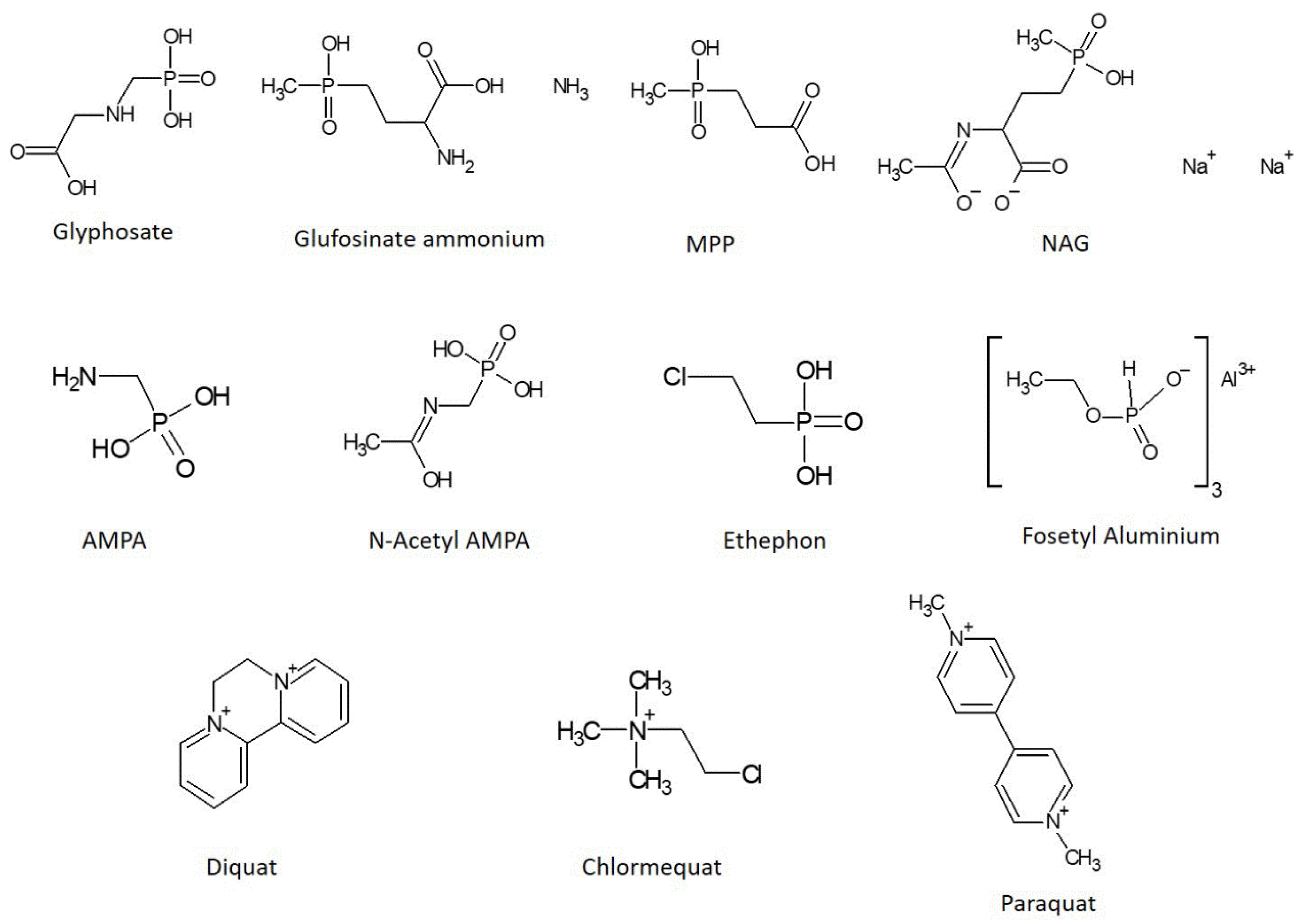
Foods | Free Full-Text | The Current Status of Analytical Methods Applied to the Determination of Polar Pesticides in Food of Animal Origin: A Brief Review

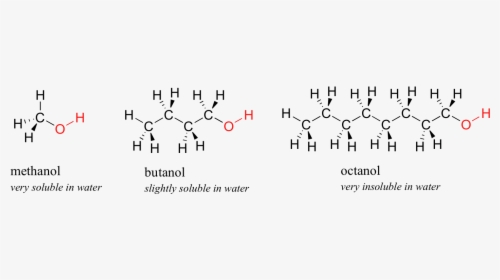
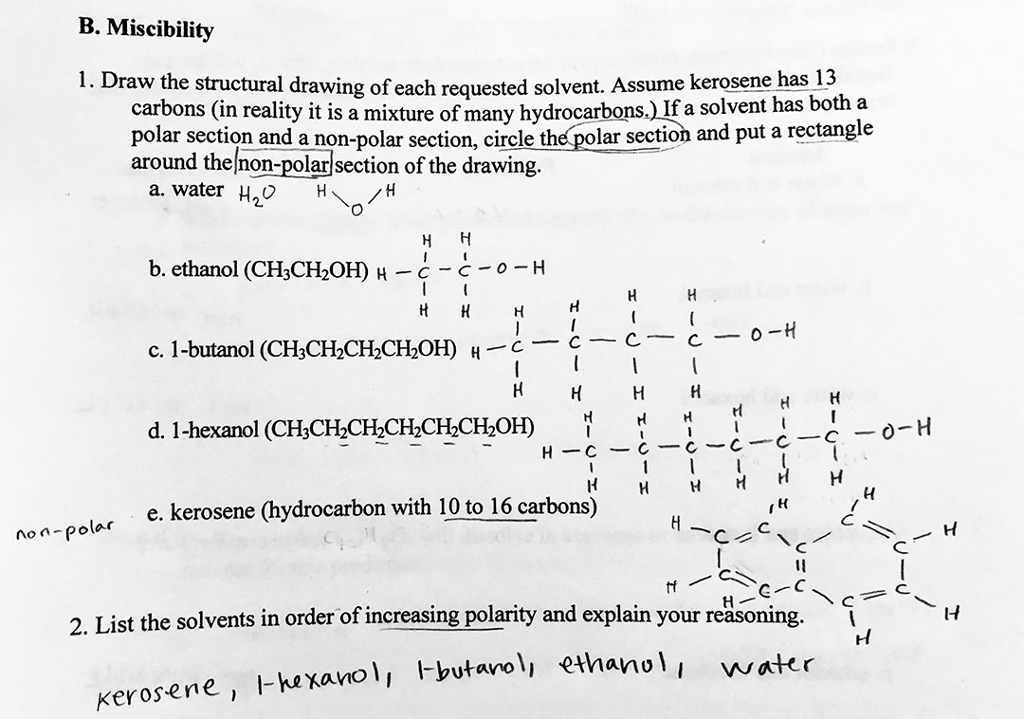
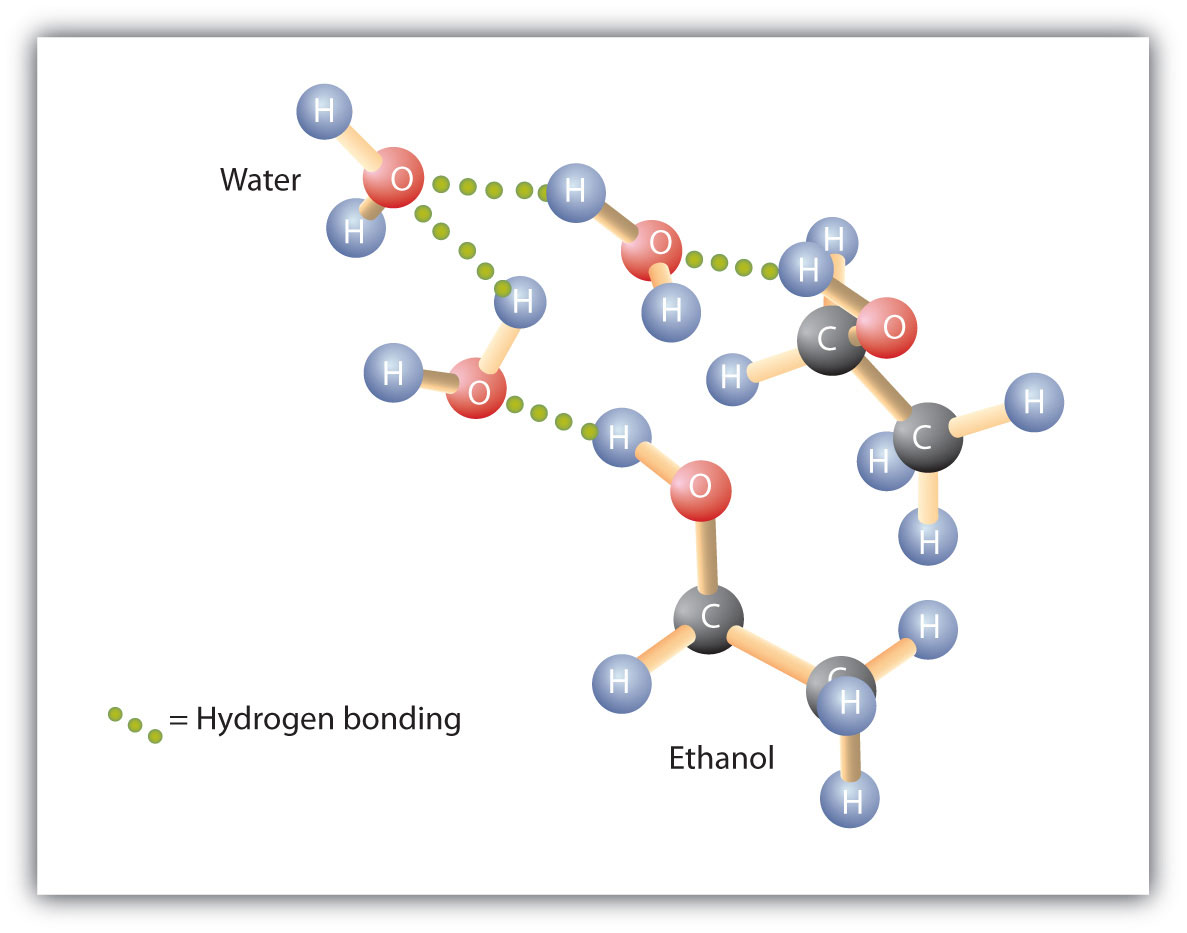
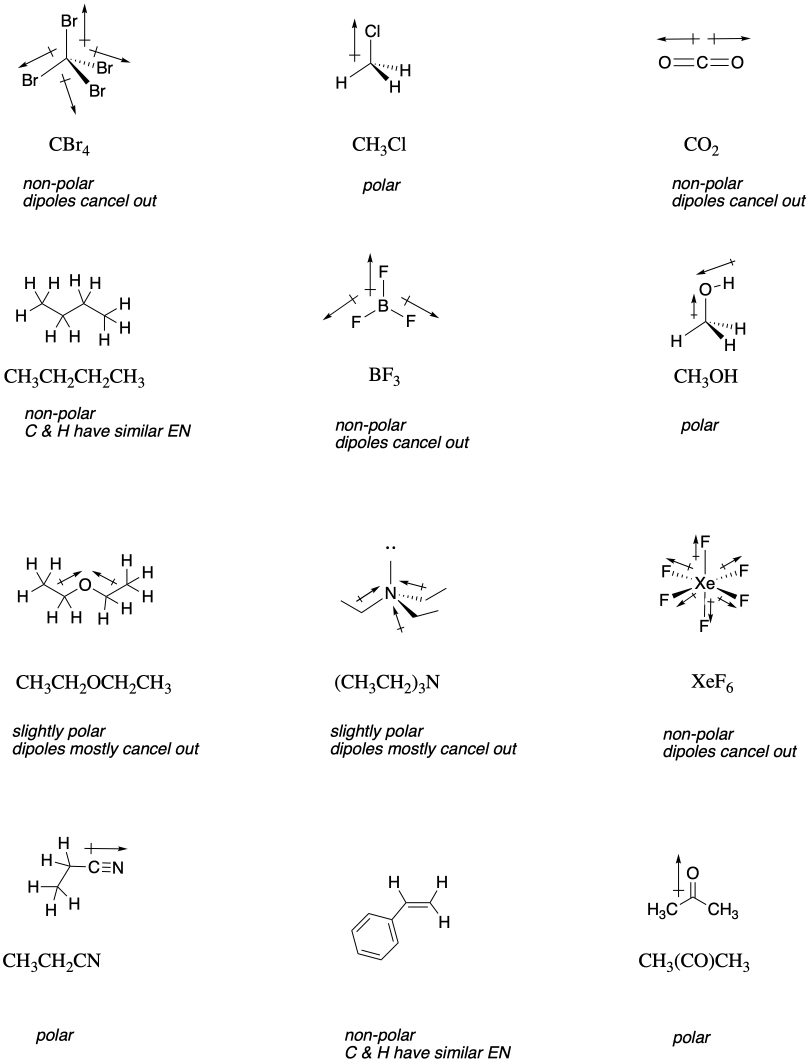
SOLVED: Ethanol is soluble in both water and toluene. Explain why this is unusual behavior: Use the concept of polarity in your answer: Explain why the solubility of I-pentanol in water at

n‐Butanol: An Ecologically and Economically Viable Extraction Solvent for Isolating Polar Products from Aqueous Solutions - Reetz - 2021 - European Journal of Organic Chemistry - Wiley Online Library

Is 1-butanol an ionic, molecular nonpolar, or molecular polar compound? What intermolecular forces are present? | Homework.Study.com
![PDF] Absorption of Carbon Dioxide into Polar Solvents of 2-Amino-2-Methyl-1-Propanol | Semantic Scholar PDF] Absorption of Carbon Dioxide into Polar Solvents of 2-Amino-2-Methyl-1-Propanol | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/678ef636c67b1127de337d0354c89fcba57cb87f/4-Table3-1.png)
PDF] Absorption of Carbon Dioxide into Polar Solvents of 2-Amino-2-Methyl-1-Propanol | Semantic Scholar