Determination of sodium carbonate and sodium bicarbonate in a solution containing both salts | BPC2113 - General Chemistry - IMU | Thinkswap

APP_H2: Fractional determination for mixture of sodium hydroxide and sodium carbonate|HIRANUMA Co., Ltd.

experimental chemistry - Equivalence point of titration of Sodium carbonate - Chemistry Stack Exchange

400 ML Solution Of Carbonate (density, Contains 22g Of Sodium Calculate The Mass Per Cent Of Sodium Carbonate In | Sodium Percarbonate 400 Gr | tk.gov.ba

SOLVED: Calculate alkalinity (mmol/L) of water to which sodium carbonate (Na2CO3) was added so that its concentration is 1.9 g/L and pH = 8.5. Dominant form of carbonate at this pH= HCO-3

SOLVED: Calculate the pH of an aqueous solution that contains 0.392 M sodium carbonate and 0.177 M sodium hydrogen carbonate. pH =
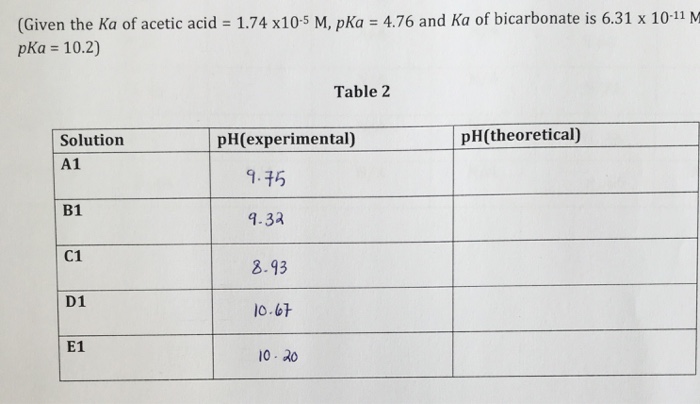
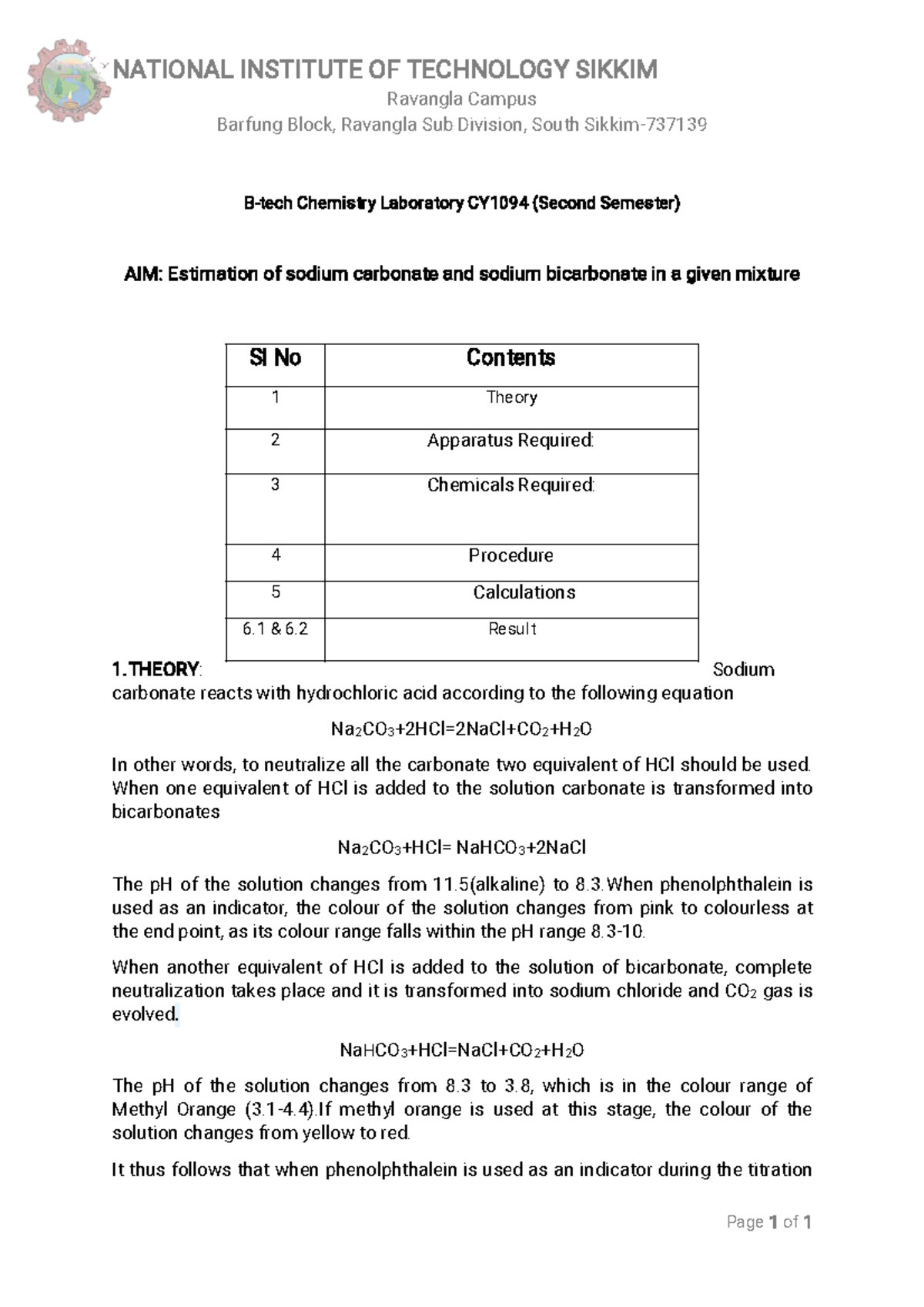
page 1 of 3 pH Experiment 1 Chemistry 201 Laboratory Summer, 2008 EXPERIMENT: DETERMINATION OF SODA ASH INTRODUCTION The meas
![SOLVED:A sodium hydrogen carbonate -sodium carbonate buffer is to be prepared with a pH of 9.40 . (a) What must the [HCO3^-] /[CO3^2-] ratio be? (b) How many moles of sodium hydrogen SOLVED:A sodium hydrogen carbonate -sodium carbonate buffer is to be prepared with a pH of 9.40 . (a) What must the [HCO3^-] /[CO3^2-] ratio be? (b) How many moles of sodium hydrogen](https://cdn.numerade.com/previews/ef6e1b45-03cf-464d-9e55-544d05108206_large.jpg)
SOLVED:A sodium hydrogen carbonate -sodium carbonate buffer is to be prepared with a pH of 9.40 . (a) What must the [HCO3^-] /[CO3^2-] ratio be? (b) How many moles of sodium hydrogen

During the titration of sodium carbonate with H Cl, the dissolved carbonate ion will exist in three different forms; CO_3^{-2}, H CO_3^{-1}, and H_2 CO_3. During which part of the titration (initial,

Identify the acid and base which form sodium hydrogen carbonate. Write chemical equation in support of your answer. State whether this compound is acidic, basic or neutral. Also write its pH value.

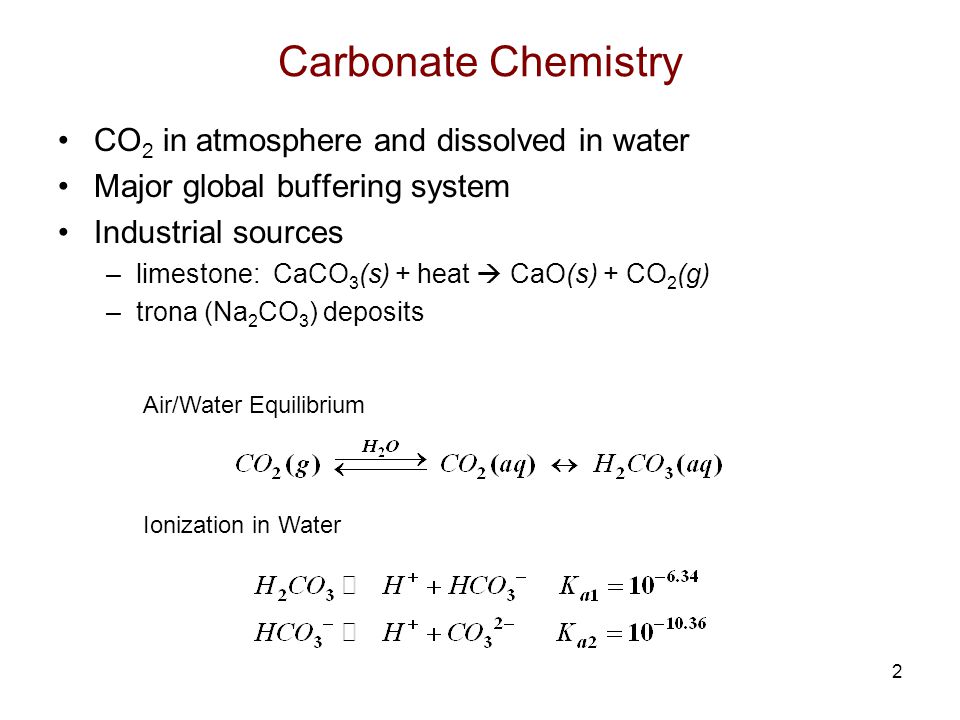



![PDF] A sodium carbonate-bicarbonate buffer for alkaline phosphatases. | Semantic Scholar PDF] A sodium carbonate-bicarbonate buffer for alkaline phosphatases. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/aaa98c1f9de4c398b6a057bf4936ea76736034c7/1-Table1-1.png)