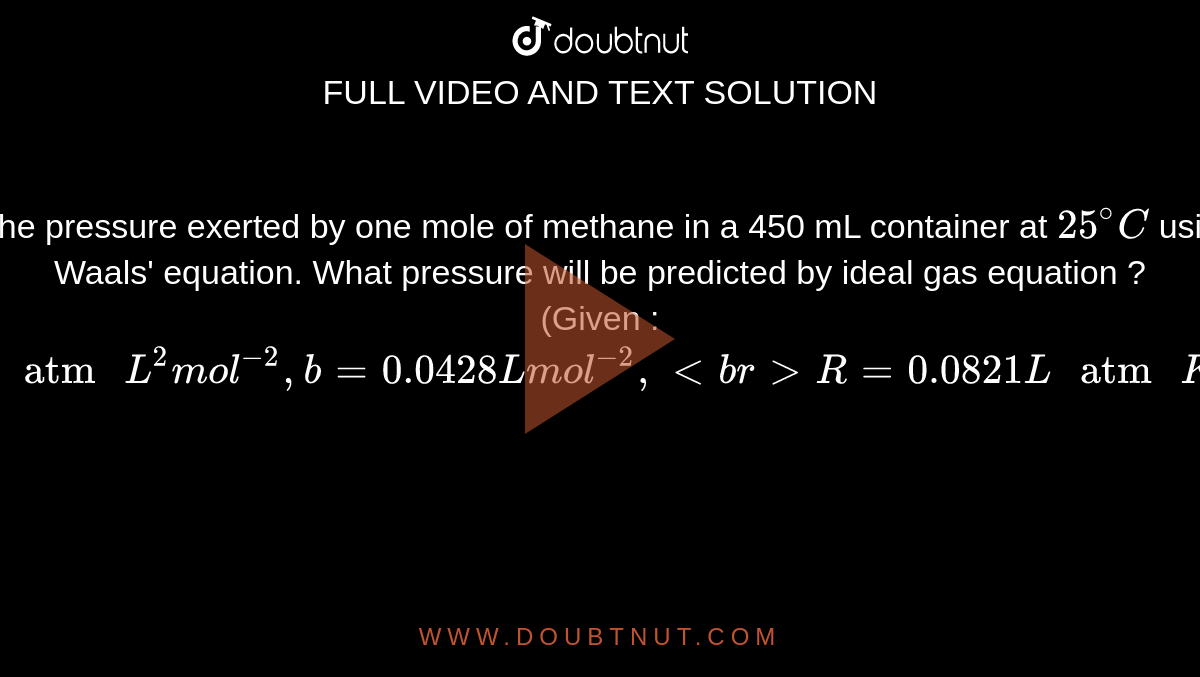
Calculate the pressure exerted by one mole of methane in a 450 mL container at 25^@C using van der Waals' equation. What pressure will be predicted by ideal gas equation ? (Given :
Five moles of ideal gas expand isothermally and reversibly from pressure 10 ATM to 2 ATM at 300 K. What is the largest mass which can be lifted through a height of

Question Video: Calculating 𝐾_𝑝 at Equilibrium for a Mixture of Nitrogen, Hydrogen, and Ammonia | Nagwa

Calculate the number of moles of gas present in the container of volume 10 L at 300 K. If the manometer containing glycerin shows 5 m difference in level as shown diagram.